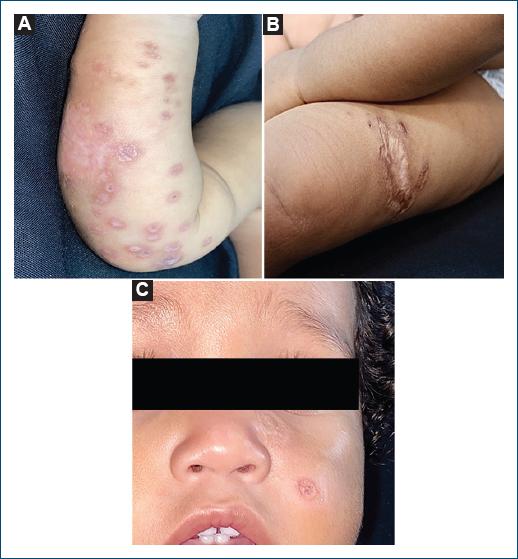
A 5-month-old male toddler from Óbidos, Brazil, presented progressive erythematous-brown plaques and papules on the face and limbs since he was 2 weeks old (Figs. 1A, B and C). Leishmania amastigote was confirmed through a skin biopsy (Fig. 2), being confirmed Leishmania Viannia guyanensis in DNA amplification technique using polymerase chain reaction.
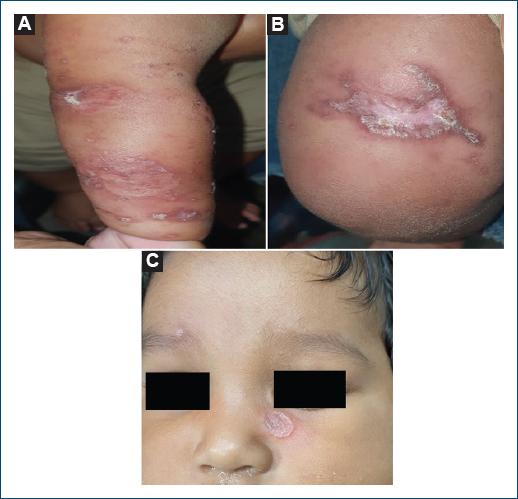
Figure 1 Lesions observed in the first outpatient visit. A: erythematous violaceous ulcerative plaques with crusts on the right arm. B: infiltrated and ulcerated plaque with erythematous violaceous edges on the left thigh. C: erythematous framed ulcerated lesion on the malar region.
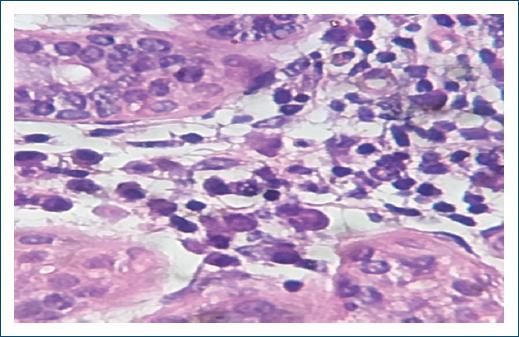
Figure 2 Histopathology from a skin lesion on the right arm demonstrating vacuolated histiocytes containing amastigotes of leishmania inside (H&E 100×).
Initial treatment with intravenous pentavalent antimonial 1 mg/kg/day caused fever and tonic-clonic seizures, leading to a switch to pentamidine 4 mg/kg intramuscularly once a week for 3 weeks. The patient showed satisfactory resolution of symptoms 1 week after the last dose of pentamidine. The skin lesions evolved as definitive atrophic scars after the treatment (Fig. 3A, B and C).
Cutaneous leishmaniasis (CL) has diverse clinical presentations and can be challenging when the clinical presentation is different from the classic ulcerated form1. In the neonatal period, CL often mimics other conditions, such as histiocytosis, lymphomas, and syphilis2,3. CL commonly affects children aged 2-12 years, corresponding to 10% of cases in endemic areas2,4.
Treatment of pediatric CL has higher rates of therapeutic failure compared to adults, depending on differences in the immune response and medication tolerance that contribute to this disparity4. In addition, children have poor tolerance to systemic medications, which may be common and potentially serious adverse events4,5.
Combination therapies, such as paromomycin, imiquimod, and amphotericin B, are being studied for optimal outcomes and reduced side effects5. The use of pentamidine for L. guyanensis infections is recommended, although off-label for children under 2 years old.
Although the reported case showed positive response and tolerability to pentamidine, further research is needed to improve CL treatment and minimize complications, aiming to reduce deformities and risks for affected patients.