Introduction
Acne vulgaris (hereafter referred to as “acne”) is one of the most common inflammatory skin disorders affecting approximately 650 million people worldwide1. It can occur in individuals of all ages, from newborns to adulthood, with its peak prevalence between the ages of 15 and 20. Although previously considered a transient adolescent state, it has been recognized that it commonly lasts longer into adulthood, especially in women2. Acne is a chronic, multifactorial, inflammatory, and mostly self-limited disorder of the pilosebaceous unit. It may present as both inflammatory (papules and pustules) and non-inflammatory (comedones) lesions on the face, the most frequent location, but also the trunk, back, and upper arms3. Although there is no mortality associated with acne, it has been remarkably associated with severe impact in physical and psychological morbidity through permanent scarring, poor self-image, depression, and anxiety4. When measured by disability-adjusted life years, acne holds the second-highest position among all skin diseases worldwide in terms of the burden it imposes5.
Concerning pathogenesis, comedones and pustules result from increased sebum production with hypertrophy of the sebaceous glands (due e.g. to androgens binding to the gland receptors). There is also hyperkeratinization of the epithelial cells of the follicular isthmus, leading to sebaceous follicle obstruction, which results in sebum accumulation. When these distended follicles rupture, the content is released into the dermis, including pro-inflammatory chemicals, which stimulate inflammation. Moreover, bacteria such as Cutibacterium acnes, Staphylococcus epidermis, and Malassezia furfur are known to induce inflammation and follicular epidermal proliferation and thereby contribute to the pathogenesis of acne3.
Recent studies have shown a correlation between acne and insulin resistance, but its role in the pathogenesis of this skin disorder is not clearly understood. However, both conditions share the same signal transduction pathways through the mammalian target of rapamycin kinase 1 (mTORC1) and insulin-like growth factor (IGF)-1. Insulin resistance, and consequently hyperinsulinemia, is responsible for increasing the proliferation and dysfunction of keratinocytes by decreasing IGF-1 binding protein (IGFBP-3), which causes a significant rise in free IGF-1 serum levels. These elevations in serum IGF-1 levels and hyperinsulinemia promote androgens synthesis, leading to subsequent abnormal sebum production, hyperproliferation of sebocytes, and lipogenesis6-9.
There are many other known factors contributing to the aggravation of acne. Among them, high glycemic food, such as refined sugars, chocolates, dairy products (also containing hormones), and junk food, were proven to stimulate follicular epidermal proliferation, through the production of IGF1-3,10.
Metformin is an oral antihyperglycemic drug used in the treatment of various conditions but mostly for Type 2 diabetes management. Its mechanism of action includes reducing basal and post-prandial plasma glucose by decreasing intestinal absorption and hepatic glucose output through gluconeogenesis inhibition. Metformin also increases insulin-mediated glucose utilization in peripheral tissues, such as muscle and liver, particularly after meals, improving insulin resistance. As a result of the improvement in glycemia, serum insulin concentrations decline slightly and so do the levels of IGF-1. There is also a decrease in food intake, body weight, and serum lipid concentrations11-13.
Metformin has been shown to be an effective and safe option for the treatment of acne in women with polycystic ovary syndrome (PCOS). The rationale relies on the insulin resistance and hyperandrogenism that are characteristic of PCOS. Thus, such patients can benefit from the insulin resistance-lowering action and, consequently, from the reduction in insulin and IGF-1 serum levels, as provided by metformin14-20. Metformin has also been suggested for the treatment of other skin disorders that are also associated with insulin resistance such as acanthosis nigricans, hidradenitis suppurative, and hirsutism13.
The aim of this evidence-based review is to collect and synthesize updated information about the use of metformin in the treatment of acne in adolescents and adults without PCOS.
Review
Materials and methods
This literature review began by developing the study question according to the population, intervention, control, and outcomes (PICO) methodology:
− Population: adolescents and adults with acne without PCOS;
− Intervention: use of metformin as an acne treatment (in monotherapy or adjuvant treatment);
− Control: no treatment or other treatment;
− Outcome: efficacy of treatment (reduction of the number and severity of acne-related lesions, duration of treatment, and associated complications).
In March of 2024, research was conducted in Medline/PubMed, Google Scholar, Cochrane Library, and SciELO with no date restrictions. The authors included meta-analyses, systematic reviews, original studies, and clinical guidelines, in Portuguese, Spanish, or English language. The exclusion criteria consisted of people with PCOS, children, and articles with languages different from the ones previously listed. The MeSH terms used for this search were: “Acneiform eruptions” and “Metformin”.
This first search found 147 publications, which were independently reviewed by the authors, based on the title and abstract, and categorized as relevant, unsure, or irrelevant. 11 duplicate articles were removed. Trials categorized as irrelevant by three or more authors were excluded as well as studies with patients diagnosed with PCOS. In this phase, 126 studies were excluded.
The next screening phase included independent analysis of the full text from the remaining 10 studies. Four studies were eliminated at this phase: a study unavailable for full reading and three other studies that didn’t fit the selected PICO method.
From the six remaining articles, five were original studies and one was a systematic review, which was excluded because it referred to results from two of the other original studies selected.
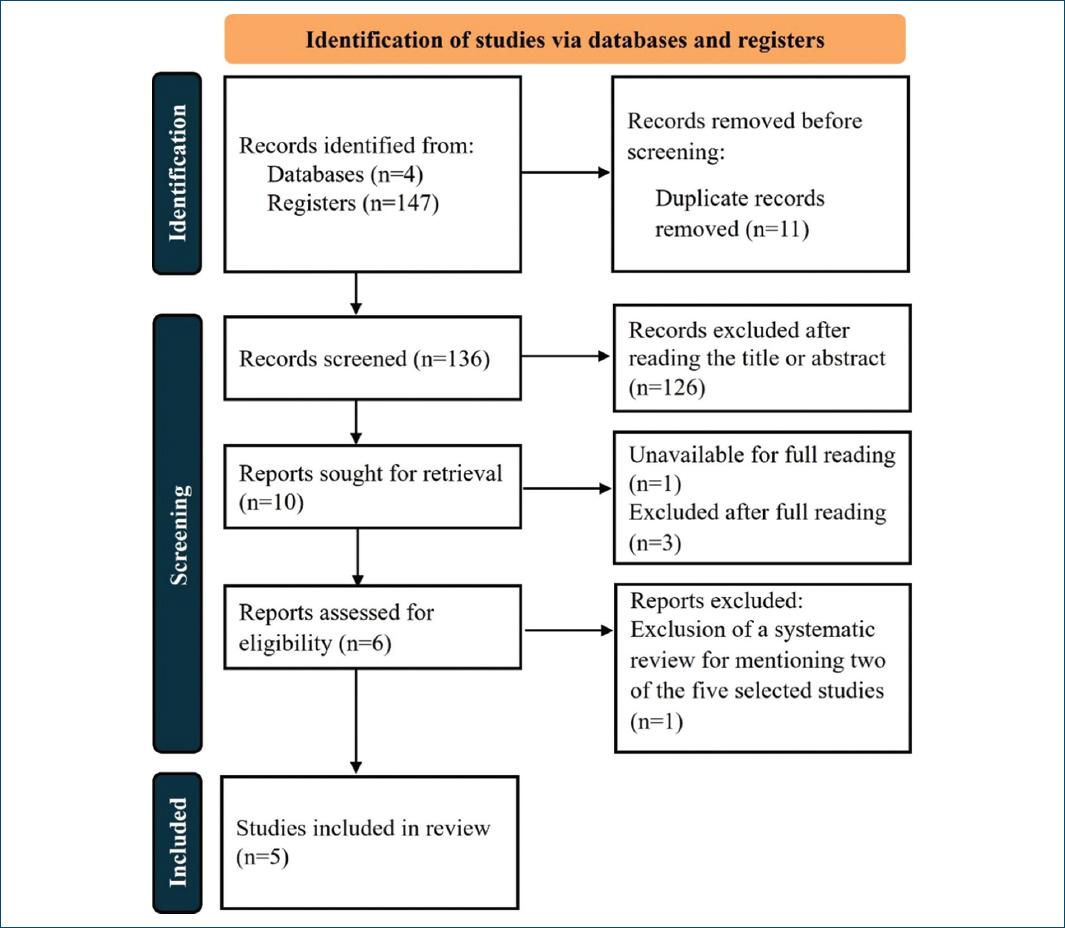
In the end, the authors analyzed five studies in this review. Two main authors independently extracted the following data for the eligible trials: author information, study design, sample size, inclusion and exclusion criteria, dosage of metformin, other concomitant treatment, follow-up duration, outcomes and results of the study (with detailed data about the acne lesions and severity). Detailed information on the selection process of the articles is represented below (Fig. 1). The strength of recommendation taxonomy (SORT) scale of the American family physicians was used to assign evidence levels and strength of recommendation grades to each one of them.
The methodology of our review is summarized according to the PRISMA 2020 flow diagram in figure 1.
Results
Brief explanation of scales applied in the studies
There are several grading systems currently available for evaluating the severity of acne. The global acne grading system (GAGS) considers six locations on the face, chest, and upper back, with a designated factor for each location based on the surface area, distribution, and density of pilosebaceous units (two factors for the forehead, two factors for each cheek, one for the nose, one for the chin, and three for both chest and back). Each of the six locations is also graded separately on a scale from 0 to 4, with the most severe lesion within that location determining the local score (1 for ≥ 1 comedone, 2 for ≥ 1 papule, 3 for ≥ 1 pustule, and 4 for ≥ 1 nodule). The total score from each location is derived from the multiplication of the factor and the local score. The total severity score is obtained from the summation of the six regional sub-scores. This scale is accessible, easy, and quick to use21.
The Cardiff acne disability index (CADI) is a questionnaire designed to measure the quality of life of people with acne, based on the disease’s impact during the previous month. Patients answer five questions aimed at assessing their psychological and social well-being, as well as their state of mind, truncal acne, and its severity. Each question from this questionnaire is scored from 0 to 3, ranging from 0 to 15 at the end of the evaluation. A higher score is associated with a greater impairment of the quality of life22.
The investigator’s global assessment (IGA) is an evaluation of the qualitative overall acne severity, using a global assessment scale with approximately five severity grades. Each of these grades should be defined by a distinct, unambiguous, and morphologic description that minimizes interobserver variability. Photographic records of baseline and assessment time points should be taken. Success is defined as an improvement of two grades from the baseline score23.
Results from the studies
KAMBOJ ET AL., 202324
This article aims to study the effectiveness of metformin in the treatment of acne in patients without PCOS. Forty-five patients with acne were selected for this study, but only 30 completed 3 months of treatment with metformin 1000 mg daily. Using the GAGS score, the 30 patients at baseline had different severities: 15 had grade 1, 15 had grade 2, and none of their patients had grade 3 or 4 acne. Clinically, metformin significantly decreased the GAGS score from 19.8 to 13.83 (p < 0.001) after 1 month of treatment, with no significant decrease in the subsequent months. They also evaluated IGF-1 and IGF IGFBP-3 levels: metformin resulted in a significant (p = 0.03) increase in total IGF-1 after 3 months (mean plasma level 109.5 ng/mL versus 96.47 ng/mL before treatment). The difference in levels of IGFBP-3 was not statistically significant (from 3645 ng/mL to 3352 ng/mL, p = 0.14). The ratio of total IGF-1/IGFBP-3 was estimated to determine the free IGF-1.
At the hormonal level, the only results that resulted in a statistically significant change were: free androgen index with a decrease from 41 (pretreatment) to 22.75 (post-treatment) (p < 0.001); mean sex hormone-binding globulin with an increase from 15.07 (pretreatment) to 22.22 (post-treatment) (p < 0.001); dehydroepiandrosterone sulfate, with an increase from 213.6 (pre-treatment) to 251.3 (post-treatment) (p < 0.01). The levels of testosterone and HOMA-IR index decreased and the level of insulin increased. However, none of these parameters had statistical relevance (p = 0.31; p = 0.70; p = 0.56, respectively).
At the genetic level, the expression of some genes changed after treatment with metformin, such as FOXO1, HMGCR, SQLE, and ACSL5, which were significantly downregulated (p = 0.006, p < 0.001, p = 0.03 and p = 0.03, respectively). The expression of IGF-1R, PPARG, SREBP1, and MTOR were not significantly changed. Metformin did not significantly change the expression of IGF-1R protein levels in sebaceous glands, keratinocytes, and eccrine glands after the 3 months of treatment.
No side effects were reported with the use of metformin.
SADATI ET AL., 202325
This study was conducted to find an alternative to doxycycline, a treatment often prescribed for acne, with few side effects. Metformin had already been successfully used in patients with PCOS and since it plays a role in reducing insulin resistance, was thought to be helpful in treating acne. Therefore, this clinical trial enrolled 45 patients but only 40 (35 women and five men) finished the entire proposed protocol (five were lost during follow-up). The patients presented moderate acne and were aged between 15 and 38 years (mean age of 24.82 ± 7.31 years). The study compared the efficacy of metformin 500 mg twice daily and doxycycline 100 mg once daily in treating acne. In both groups, the patients also applied 5% benzoyl peroxide gel topically every night over the lesions for 30 min. The treatment and follow-up lasted 2 months, during which patients were visited monthly for assessment of the results.
This study concluded that metformin had equal efficacy in reducing acne severity at month two when compared with doxycycline and both are effective in reducing acne severity and overall number of lesions. In terms of the scales used to assess the improvement of acne lesions in IGA, CADI, total lesion count, and the number of non-inflammatory lesions, there was a significant reduction in both groups (p < 0.001) with no significant difference between them (p > 0.05). Although both treatments presented a reduction in the GAGS scale and the number of inflammatory lesions, doxycycline had a slightly better response than metformin ([F (1.71-452.60) = 5.85, p = 0.007] and [F (1.37-518.15) = 3.73, p = 0.046], respectively).
Regarding side effects, in the group using metformin, three patients reported mild to moderate gastrointestinal discomfort, especially when taken on an empty stomach. In the doxycycline group, one patient reported mild photosensitivity.
ALBALAT ET AL., 202226
This study conducted in Egypt aimed to evaluate the role of metformin in the treatment of acne by reducing the level of IGF-1. This cohort study included 50 patients (33 women and 17 men) with acne resistant to other therapies. The severity of acne was categorized as follows: Five patients (10%) had mild acne, 34 patients (68%) had moderate acne and 11 patients (22%) had severe acne. Their ages ranged from 16 to 30 years, with a mean age of 18.7 ± 3.1 years. The patients were treated with metformin 500 mg twice daily for 4 months. Patients were excluded if they presented any medical conditions that could affect IGF-1 levels, such as growth hormone disruption, diabetes mellitus, pregnancy, or lactation.
Importantly high serum IGF-1 levels were detected in acne patients, not significantly correlated with acne severity but positively correlated with body mass index (BMI). Furthermore, higher BMI was associated with higher acne scores and severity at baseline. A statistically significant decrease in IGF-1 levels (45.4 ± 6.3 vs. 28.8 ± 8.2 [p = 0.001]) and BMI values (23.5 ± 1.8 vs. 22.3 ± 1.3 [p = 0.001]) was observed after the course of treatment with metformin. Clinically, a significant improvement in acne severity was also observed, from baseline to month 4, as demonstrated by the reduction in acne score (25.2 ± 6.8 to 13.6 ± 4.5; p = 0.001) and acne grading (mild: 5 (10%) to 40 (80%); moderate: 34 (68%) to 10 (20%); severe: 11 (22%) to 0; p < 0.0001).
During the study, six patients reported mild gastrointestinal discomfort, nausea, and vomiting and three patients reported hypoglycemia. The author’s strategy to improve side effects was temporary dose reduction until symptom improved. No time or dose reduction was explained in the article.
ROBINSON ET AL., 201927
This study conducted in dermatology clinics of the University of Malaya Medical Centre and Hospital Kuala Lumpur in Malaysia represents the first one to evaluate the efficacy of metformin as an adjunct therapy for acne in a healthy population irrespective of sex, insulin resistance status, and BMI. A total of 84 patients (59 women and 25 men) aged 18-39 years (mean age of 22.9 ± 4.34 years) were randomized to receive treatment with (n = 42) or without (n = 42) metformin 850 mg daily (both groups received oral tetracycline 250 mg bid + topical benzoyl peroxide 2.5% gel). The exclusion criteria included patients with acne conglobate, acne fulminans, secondary acne, pregnant or breastfeeding women, and people on topical anti-acne preparations, chemical peels, or systemic acne therapies. Patients were evaluated at baseline, week 6, and week 12, by IGA grading, CADI scoring, and acne lesion count, as well as measurements of blood pressure, pulse rate, and weight. In each visit, pills were counted and the topical preparations were inspected, ensuring adherence to the study. Seventy-six patients completed the proposed protocol (six were lost during follow-up and two for adverse reactions, one to tetracycline, and the other to benzoyl peroxide).
Participants who received metformin as an adjuvant treatment yielded higher treatment success rates than those who did not have metformin (66.7% vs. 43.2%, p < 0.05). There was a greater mean percentage reduction from baseline in total lesion counts in the metformin group than in the non-metformin group at 12 weeks (−71.4% vs. −65.3%, p = 0.278), as well as in non-inflammatory and inflammatory lesions (−44.9% vs. −37.4% [p = 0.445] and −83.1% vs. −75.63% [p = 0.064], respectively) and also a greater reduction in the CADI score (4.82 ± 3.39 vs. 4.22 ± 3.56, p = 0.451), even though all of these parameters were statistically non-significant.
Metabolic parameters were also evaluated proving that metformin was equally efficient in improving acne, with a significant reduction of 0.26 ± 0.72 kg/m2 in the intervention group compared to an elevation of 0.3 ± 0.72 kg/m2 in the control group (p < 0.05). The intervention group was further analyzed based on BMI categories: lean and overweight. Both categories yielded similar improvements in lesion counts at week 12 (−70.2% vs. −73.7%, p = 0.558), with 61.5% achieving treatment success in the lean group and 76.9% in the overweight group (p = 0.346). Therefore, this study showed that metformin is able to improve acne irrespective of BMI.
Regarding side effects, in the control group, one patient developed secondary gastroenteritis to tetracycline and another developed severe erythema to benzoyl peroxide. Both dropped out of the study. In the metformin group, 13 patients reported gastrointestinal symptoms such as abdominal discomfort and bloating, nausea, and vomiting, but all thirteen completed the proposed protocol. No patient reported hypoglycemia.
FABBROCINI ET AL., 201628
This study was conducted to assess if the use of metformin combined with a low-calorie diet is effective in reducing the severity of acne in patients with an altered metabolic profile and insulin resistance. The investigators assessed 42 young male patients with acne resistant to common standard therapies. The inclusion criteria were: age between 17 and 24 years, male sex, and presence of acne for at least 1 year that was resistant to common therapies. Those with other dermatological or endocrinological disorders were excluded. A total of 20 men with an abnormal metabolic profile (defined as impaired fasting glucose, raised levels of total and low-density lipoprotein cholesterol, reduced levels of high-density lipoprotein cholesterol, and waist circumference and BMI at the upper limit of normal) were selected and randomly assigned in a 1:1 ratio into two groups (A and B). All patients completed the proposed protocol.
Participants of group A received treatment with metformin (500 mg, twice daily) and a low-calorie diet (1500-2000 kcal, rich in fruits, vegetables, and fish, low in carbohydrates), in addition to daily topical symptomatic care (bland detergent and a sebostatic cream based on azelaic acid and nicotinamide). In group B, daily topical symptomatic care was maintained but no metformin or change in diet was prescribed. Patients were assessed at baseline (T0) and at 6 months (T1).
BMI and waist-to-hip ratio (WHR) in both groups were positively correlated with GAGS at T0: the higher the BMI and WHR value, the higher the GAGS (p = 0.02 and p = 0.04, respectively). In group A, there was a statistically significant reduction in GAGS from 25.1 ± 8.9 at T0 to 14.1 ± 10.4 at T1 (p < 0.03), which was not demonstrated in group B (p = 0.06).
The oral glucose tolerance test at 120 min also decreased significantly in group A: from 88.2 ± 8.1 at T0 to 79.2 ± 6.9 at T1 (p = 0.04). There was also a statistically significant positive correlation between T1 and T0 for HOMA-IR and GAGS (p < 0.03) in this group. The difference between pre-treatment and post-treatment HOMA-IR between the two groups was statistically significant (p < 0.001).
The authors concluded that the improvement in acne and insulin sensitivity after the use of metformin combined with a low-calorie diet suggests a new possible approach to acne therapy, particularly in male subjects with acne resistant to other therapies and altered metabolic profile. No side effects from metformin use were reported during the study.
These articles are summarized in tables 1 and 2.
Table 1 Characteristics of the five clinical studies using metformin in acne treatment, type of studies, and population involved
| Study | Kamboj et al., 2023 24 | Sadati et al., 2023 25 | Albalat et al., 2022 26 | Robinson et al., 2019 27 | Fabbrocini et al., 2016 28 |
|---|---|---|---|---|---|
| Type of study | Observational study | Randomized controlled trial | Cohort study | Prospective randomized open labeled study | Randomized controlled trial |
| Methods | Duration: 3 months Assessment: baseline + M1, M2, M3 | Duration: 2 months Randomization 1:1 Assessment: baseline + M1, M2 Blinded by a dermatologist | Duration: 4 months Assessment: baseline + M4 | Duration: 12 weeks Randomization 1:1 Assessment: baseline + Wk6, Wk12 | Duration: 6 months Randomization 1:1 Assessment: baseline + M6 |
| Patients | (n = 30) No more information | (n = 40) 35 women, 5 men 15-38 years | (n = 50) 33 women, 17 men 16-30 years | (n = 84) 59 women, 25 men 18-39 years | (n = 20) Males only 17-24 years |
| Type of acne | Different degrees of acne severity | Moderate acne | Acne resistant to other therapies for 6 months | Moderate to severe acne and: IGA score ≥ 3 ≥ 20 inflammatory lesions ≥ 30 non-inflammatory lesions ≤ 5 nodulocystic lesions | Resistant acne + altered metabolic profile |
| Exclusion criteria | No information | Systemic treatment in the past month; topical treatment in the past 2 weeks; systemic diseases; concomitant drugs that interact with metformin or doxycycline; contraceptive medications; pregnant or lactating women; women with PCOS | Medical conditions that could affect IGF-1 levels | Acne conglobate/fulminant; secondary acne; pregnancy/breastfeeding; topical anti-acne preparations; chemical peels; systemic acne therapies | Other dermatological or endocrinological diseases |
| Intervention | Metformin 1000 mg id | Metformin 500 mg bid + BPO 5% gel + SPF > 30 sunscreen | Metformin 500 mg bid | Metformin 850 mg id + oral tetracycline 250 mg bid+BPO 2.5% gel | Metformin (500 mg bid) + low-calorie diet + topical treatment |
| Control | No control group | Oral doxycycline 100 mg id + BPO 5% gel + SPF > 30 sunscreen | No control group | Oral tetracycline 250 mg bid + topical BPO 2.5% | Topical treatment |
Bid: twice daily; BPO: benzoyl peroxide; id: once daily; IGA: investigator’s global assessment; IGF-1: insulin-like growth factor-1; M1: month 1; M2: month 2; M3: month 3; M6: month 6; PCOS: polycystic ovary syndrome; SPF: sun protection factor; Wk6: week 6; Wk12: week 12.
Table 2 Clinical and laboratorial outcomes and main results of the five clinical studies using metformin in acne treatment
| Study | Kamboj et al., 2023 24 | Sadati et al., 2023 25 | Albalat et al., 2022 26 | Robinson et al., 2019 27 | Fabbrocini et al., 2016 28 |
|---|---|---|---|---|---|
| Clinical outcomes | Improvement in GAGS score | Improvement in GAGS, IGA, CADI, total lesion count, number of lesions | Improvement in GAGS and acne score; improvement on BMI | Improvement in lesion counts, IGA, and CADI; metabolic parameters | Improvement in GAGS, BMI, WHR, HOMA-IR |
| Laboratory outcomes | Improvement of insulin, testosterone, SHBG, DHEA-S, and HOMA-IR; Assess of IGF-1, IGFBP-3 levels and the ratio of total IGF-1/IGFBP-3; Evaluate the expression of genes related to acne’s pathophysiology | None | Improvement of IGF-1 level | None | Improvement in HOMA-IR; improvement in fasting glucose, fasting insulin, OGGT, total cholesterol, HDL cholesterol |
| Main results | GAGS: significant decrease from 19.8 to 13.83 (p < 0.001) at M1; no significant decrease in the mean score at M2 and M3; Increase in total IGF-1 at M3 | At M2 GAGS: 10.65 ± 6.29 versus 14.95 ± 12.89; IGDMC: p = 0.188; significant reduction in the control group (F (1.71-452.60) = 5.85, p = 0.007) IGA: 1.50 ± 0.51 vs. 1.60 ± 0.50; IGDMC: p = 0.537 CADI: 6.05 ± 2.81 vs. 6.40 ± 2.11; IGDMC: p = 0.659 Total lesion count: 26.05 ± 16.57 vs. 33.20 ± 29.81; IGDMC: p = 0.354 Number of inflammatory lesions: 10.35 ± 9.49 vs. 16.10 ± 16.86; IGDMC: p = 0.694; significant reduction in the control group (F [1.37-518.15] = 3.73, p = 0.046) Number of non -inflammatory lesions: 14.95 ± 12.64 vs. 17.60 ± 22.02; IGDMC: p = 0.279 | At M4 Acne score: 25.2 ± 6.8 vs. 13.6 ± 4.5 (p = 0.001) Acne grading: Mild: 5 (10%) vs. 40 (80%) (p < 0.0001) Moderate: 34 (68%) vs. 10 (20%) (p < 0.0001) Severe: 11 (22%) vs. 0 (p < 0.0001) | Wk12 Non-inflammatory lesion count: −44.9% vs. 4% (p = 0.445) Inflammatory lesion count: −83.1% vs. 6% (p = 0.064) Total lesion count: −71.4% vs. 65.3% (p = 0.278) Treatment success rate (IGA score 0 or 1 or improvement of ≥ 2 grades): 66.7% vs. 43.2% (p = 0.04) CADI score: 4.82 ± 3.39 vs. 4.22 ± 3.56 (p = 0.451) | At M6 GAGS: significant decrease with intervention from 25.1 ± 8.9 to 14.1 ± 10.4 (p < 0.03); no significant decrease in control group from 24.9 ± 7.6 to 19.4 ± 7.4 (p = 0.06) |
| Safety | No side effects were reported | Mild-to-moderate GIT discomfort with metformin (n = 3) mild photosensitivity with doxycycline (n = 1) | Mild GIT symptoms (n = 6) and mild hypoglycemia (n = 3) with metformin | GIT symptoms with metformin (n = 13) Gastritis with tetracycline (n = 1) severe erythema to BPO (n = 1) | No side effects were reported |
| Level of evidence | 2 | 2 | 2 | 2 | 2 |
BMI: body mass index; CADI: Cardiff acne disability index; DHEA-S: dehydroepiandrosterone sulfate; GAGS: global acne grading system; GIT: gastrointestinal tract; HDL: high-density lipoprotein; HOMA-IR: homeostasis model assessment insulin resistance; IGA: investigator’s global assessment; IGDMC: intergroup difference in the mean change; IGF-1: insulin-like growth factor-1; IGF-1R: insulin-like growth factor-1 receptor; IGFBP-3: IGF binding protein 3; M1: month 1; M2: month 2; M3: month 3; M6: month 6; OGGT: oral glucose tolerance test; PCOS: polycystic ovary syndrome; SPF: sun protection factor; SHBG: sex hormone-binding globulin; WHR: waist to hip ratio; Wk12: week 12.
Discussion
Acne is a very common inflammatory skin disorder that manifests through the formation of comedones and pustules, resulting in varying degrees of severity and discomfort for affected individuals. Its pathogenesis is not fully understood, but it probably involves a complex interplay of mechanisms, including hypertrophy of the sebaceous glands with excess sebum production, hyperkeratinization of the epidermis epithelial cells leading to clogged pores, bacterial proliferation (mostly C. acnes, S. epidermis, and M. furfur) and inflammation1,3,10. The role of high glycemic food and insulin resistance has also been discussed. It has been postulated that both acne and insulin resistance share the same signal transmission paths, using the mTORC1 and IGF-1, with hyperinsulinemia causing elevation in IGF-1 serum levels. In addition, hyperinsulinemia promotes androgens synthesis and subsequent hyperseborrhea1-3,6.
Metformin is a very well-known hypoglycemic agent, a mTORC1 inhibitor drug, that may lead to an improvement in acne development and appearance24,27,29, not only by reducing IGF-1 serum levels but also by having an anti-inflammatory and anti-androgenic action29,30.
In this review, aimed at patients with acne without PCOS, all five studies demonstrated statistically significant improvement of acne lesions in the groups treated with metformin, whether as monotherapy or combined topical/systemic treatment and/or diet. However, deriving reliable conclusions proves challenging due to significant disparities among the various articles. These variances notably encompass the dosage administered, with four of the articles employing a dosage of 1000 mg of metformin per day, while one utilized 850 mg/day. Furthermore, discrepancies extend to the presence of distinct control groups, with two articles lacking such groups, while the remaining three incorporated diverse combinations of topical and oral therapies within their control measures. Moreover, methodological differences emerge, with three articles adopting a randomized controlled trial design and the remaining two employing observational methodologies.
In addition, one of the articles used a conventional therapy in the control group, specifically doxycycline. This adds complexity to assessing whether the improvement in acne lesions resulted from the intervention with metformin or from the use of doxycycline. Therefore, although individually each article demonstrated statistically significant differences in favor of the use of metformin, it is challenging to directly compare all articles.
Furthermore, conclusions differed regarding the action of metformin on IGF-1. As mentioned in acne, IGF-1 levels are increased, and metformin, as an oral antihyperglycemic drug, was expected to reduce them. This was demonstrated in Albalat’s et al. study26 where metformin was given 500 mg twice a day and the IGF-1 levels decreased. In contrast, in the Kamboj et al. study24 in which 1000 mg of metformin was administered once daily, this reduction was not observed. In fact, a statistically significant increase in IGF-1 levels was detected. Ultimately, the patients involved in four of the studies were given the same dose of metformin (1000 mg/day), raising the question of whether the diversity of dosage has a real impact on these results.
On the other hand, one of the articles achieved a statistically significant improvement in acne with a lower dosage of metformin (850 mg/day)27.
A limitation of this study is the scarcity of literature on this topic, resulting in limited data available for extraction by the authors. The included articles exhibited a restricted sample size and significant heterogeneity in population characteristics, both in age and sex, with either female predominance or exclusively males. In addition, there were differences in the dose of metformin administered and the duration of treatment across studies. This heterogeneity may have hindered the ability to detect additional benefits of metformin, underscoring the need for larger, more comprehensive studies. The authors consider that it is essential to carry out further research with high-quality, large-scale, randomized placebo-controlled trials to clarify the non-consensual findings. Clear guidelines should be issued to define how to use metformin as an acne vulgaris treatment option, whether as monotherapy or as adjuvant therapy, the dose and duration of treatment, and define the population indicated for its use.
Therefore, integrating this drug into acne treatment would represent a significant innovation, with the potential to greatly enhance quality of life and reduce acne-related morbidity. Metformin’s widespread use, established favorable safety profile, tolerability, and relatively affordable cost further underscore its potential impact11,12,31-35.
Conclusion
Metformin has shown to be a possible promising option to be considered in the treatment of acne in the absence of PCOS, reducing both the quantity and severity of acne lesions while controlling other parameters associated with the metabolic syndrome.
The authors determined the level of evidence according to the SORT scale to be grade B. Therefore, further homogeneous and higher-quality studies are needed.