INTRODUCTION
Chlorpyrifos (CLP) [O, O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate] is an organophosphorus insecticide. Currently, its use is banned throughout the European Union, but it is still used in South America and Asian countries, and with some restrictions in China and USA. It has been one of the most widely applied pesticides due to its low-cost and its high efficiency (Bose et al., 2021). CLP involves a serious risk for humans, since it is an acetyl cholinesterase (AChE) inhibitor, causing neurotoxic disorders. Nowadays its presence remains in water samples and soils even in those countries where CLP had been banned. Its continued application leads to its accumulation in soils, affecting their properties and productivity (Bose et al., 2021).
Microbial biodegradation is one of the best strategies to remove CLP from the environment. Biostimulation and bioaugmentation are used to enhance its bioremediation (Rayu et al., 2017). CLP is highly hydrophobic and persistent in soils, and bioavailability is an essential factor in efficiency of pesticide biodegradation in soils. For this reason, cyclodextrins (CDs) have been recognized as a tool for the elimination of pesticides in soils, since they are able to increase their water solubility, enhancing bioavailability to accelerate its biodegradation (Morillo et al., 2020).
The objective of this work was to bioremediate a soil contaminated with the insecticide CLP in presence of the bacterial strains B. megaterium CCLP1 or B. safensis CCLP2. Both strains were isolated from two agricultural soils treated with CLP for years. Several biodegradation treatments were performed: biostimulation, adding macro-and micronutrients solutions (NS), bioaugmentation, inoculating isolated bacterial strains, and addition of a CD as availability enhancer. Lastly, the feasibility of bioremediation strategy was verified through ecotoxicological studies at the beginning and after the CLP decontamination treatments.
MATERIALS AND METHODS
CLP was provided by Sigma-Aldrich (purity > 98%). Randomly methylated β-cyclodextrin was provide by Cyclolab (Budapest, Hungary). B. megaterium CCLP1 and B. safensis CCLP2 were isolated from agricultural soils by enrichment cultures with CLP. Soil properties of studied soil are show in Table 1.
Table 1 Some properties of the soil used
| Soil | pH | CO3 -2 (%) | OM (%) | Sand (%) | Silt (%) | Clay (%) |
| ALC | 5.1 | 0.5 | 13.9 | 69.1 | 7.8 | 23.1 |
| R | 7.7 | 4.0 | 3.4 | 77.0 | 9.5 | 13.5 |
| LL | 7.8 | 4.0 | 0.9 | 79.6 | 9.3 | 11.1 |
Biodegradation experiments were carried out in 25 mL sterilized glass vials in triplicates. Each vial contained: 1 g of contaminated soil sample (50 mg kg-1 CLP) and the required volume of NS to reach 40% of the soil water holding capacity (73.4 ml per 100 g of soil) for biostimulation. Several biodegradation strategies were designed, where contaminated soil was treated with a solution of RAMEB (an amount corresponding to 10 times that of the CLP molar soil concentration initially added) and/or bioaugmented, inoculating the isolated degrading bacteria (108 CFU g-1). Abiotic degradation control tests were also performed by adding 200 mg L-1 of HgCl2. All assays were kept at 30°C under agitation at 180 rpm during 100 d. Samples were taken at different incubation periods to monitor the concentration of CLP. 1 g of soil sample was extracted with 5 mL of acetonitrile:water (90:10). The extraction process was: 1) 1 min under vortex mixer, 2) 10 min in an ultrasound bath, 3) 1 h of shaking at 100 rpm and 20 ± 1°C, and 4) 10 min centrifugation at 8000 rpm. Residual CLP was quantified by GC/MS. The separation was achieved with a 30×0.25 mm I.D. DB-5 MS (J&W Scientific, Agilent Technologies) column, which is covered with phenyl methylpolysiloxane 5%. The analytical method used was based on the method described by Ishag et al. (2016). Biodegradation curves were adjusted to three kinetic models: First Simple Order Kinetics (SFO), Hockey-Stick Model (HS) kinetics or Multicompartmental First Order Model (FOMC), following the indications of the Focus working group.
Microtox® Test System using Vibrio fischeri was employed to measure the toxicity of bioremediated soil. Briefly, 3 mL of NaCl at 2% were added to 2 g of soil. These suspensions were shaken for 10 min, and centrifuged (2 min, 10000 rpm). Samples were serially diluted (1:2) with NaCl at 2% solution. After 15 min of exposure, the bioluminescence was read to calculate EC50 and TU. EC50 indicates the pollutant concentration (% v/v) that produce a reduction of 50% in V. fischeri luminescence, and the toxic units (TU) were calculated according to the equation TU = 100/EC50.
Toxicity was measured at the beginning and at the end of the treatment and was analysed relatively to the control.
RESULTS AND DISCUSSION
CLP biodegradation in soil
Table 2 shows the different treatments conducted to achieve an effective remediation of the soil contaminated with CLP.
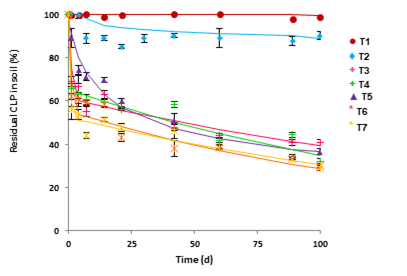
ALC soil from a Natural Park, was used to carry out biodegradation test. No significant biodegradation was observed in the non-inoculated soil in presence of its endogenous soil microbiota (0.3% after 100 d, Figure 1, T1). However, when NS was added to stimulate soil microbiota, a slight increase in the biodegradation percentage was observed (15.7%, T2), but DT50 value (required time for the pollutant concentration to decline to half of its initial value) was almost 2 years (Table 3). It indicates the high persistence of CLP in this soil. Based on these results, the application of bioremediation techniques such as bioaugmentation and the addition of RAMEB to improve the soil CLP biodegradation rate were considered.
B. megaterium CCLP1 and B. safensis CCLP2 were inoculated individually. After 100 d of inoculation, 60.6% and 64.8% of extent of degradation was reached, respectively (Figure 1, T3, T4). In addition, DT50 was significantly reduced from 696.6 d (only with biostimulation) to 44.6 and 47.1 d (Table 3) in the case of inoculation with B. megaterium CCLP1 and B. safensis CCLP2, respectively.
Table 3 Calculated kinetic parameters for CLP biodegradation in soil
| Treatment | Kinetic model | DT50 | Extent of biodegradation (%) |
| T1 | SFO | ∞ | 0.3 |
| T2 | SFO | 696.6 | 15.7 |
| T3 | HS | 44.6 | 60.6 |
| T4 | HS | 47.1 | 64.6 |
| T5 | FOMC | 38.9 | 63.6 |
| T6 | HS | 14 | 71.5 |
| T7 | HS | 7.9 | 69.6 |
RAMEB was added to ALC soil (Figure 1, T5), achieving a 63.6% of biodegradation, and a decreasing in DT50 (38.9 d), regarding NS treatment. RAMEB would be provoking an increase of the CLP fraction in the soil solution, which involves an improvement in its extent and rate of biodegradation (Köse et al., 2022). CCLP1 or CCLP2 strains, jointly applied with RAMEB, were the most effective treatments (Figure 1, T6, T7). DT50 was reduced to 14 and 7.9 d, respectively (Table 3).
Toxicity analysis in soil samples
The toxicity of the CLP and their potential formed metabolites remaining in soil were assessed at the end of the bioremediation process. Results were compared according to classification proposed by Persoone et al. (2003) (Table 4). Non-inoculated contaminated soil (T1) showed a value of TU 5.6 (1 < TU < 10, acute toxicity). However, in presence of B. megaterium CCLP1, the toxicity was undetectable after 100 d. When B. safensis CCLP2 was inoculated, the level of toxicity decreased from acute toxicity to non-toxic (TU 0,004, TU < 0.4, non-toxic). These results confirmed its ability to degrade CLP to non-toxic substances. In addition, the effectiveness of bioaugmentation and RAMEB addition (T6 and T7 experiments) was demonstrated since no toxicity was detected in any case (Table 4). It is worth noting that, as far as we know, no study of CLP ecotoxicity in soil has been published previously.
CONCLUSIONS
B. megaterium CCLP1 and B. safensis CCLP2, isolated in our lab from two agricultural soils by enrichment cultures, in presence of CLP as the only carbon source, proved to be able to degrade CLP in soil. RAMEB was used as a bioavailability enhancer of CLP in soil due to its ability to form an inclusion complex. However, the most effective treatment was the joint application of bacterial strains and cyclodextrin. The feasibility of bioremediation strategy was checked using ecotoxicological studies, showing a decline in toxicity parameters in all cases where the selected bacterial strains were used. A complete elimination in the toxicity was achieved, when bioaugmentation and CD treatments were applied, reducing also the time required to remediate the soil to less than 14 d. It is concluded that, biodegradation should be considered as a useful process to degrade CLP, but the soil particular conditions must be adjusted to achieve the most suitable for bioremediation.