Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.28 no.1 Coimbra 2010
Inhibition of the Corrosion of Zinc in 0.01 - 0.04 M H2SO4 by Erythromycin
N.O. Eddy,1,* S.A. Odoemelam,2 E.C. Ogoko,2 B.I. Ita3
1 Dept. of Chemistry, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
2 Michael Okpara Dept. of Chemistry, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria
3 Dept. of Chemistry, University of Calabar, Calabar, Cross River State, Nigeria
Abstract
Inhibition of the corrosion of zinc in 0.01 to 0.0 4 M H2SO4 by erythromycin was studied using weight loss and hydrogen evolution methods. The results obtained indicate that erythromycin is a good adsorption inhibitor for the corrosion of zinc in H2SO4 solutions. The inhibition efficiency of erythromycin increases with increasing concentration but decreases with increase in temperature. Thermodynamic and adsorption studies reveal that the adsorption of erythromycin on zinc surface is exothermic, spontaneous and is characterised with increasing degree of orderliness. The adsorption characteristics of the inhibitor are best described by the Langmuir adsorption model. From the variation of inhibition efficiency with temperature and the calculated values of the activation and free energies (which are within the limits expected for physical adsorption), we propose that the adsorption of erythromycin on zinc surface is consistent with the mechanism of physical adsorption.
Keywords: corrosion inhibition, Zn, H2SO4, erythromycin.
Introduction
Zinc is an important metal with numerous industrial applications and is mainly used for the corrosion protection of steel [1-6]. The zinc coated steel materials provide a greater resistance to corrosion but when exposed to humid atmosphere, it undergoes rapid corrosion with the formation of corrosion product known as white rust. The formation of white rust is generally observed in galvanized materials and renders the plated zinc materials unsuitable for industrial applications. Also, industrial processes such as scale removal and cleaning of zinc surfaces with acidic solutions expose zinc to corrosion. Therefore, in order to protect the metal from corrosion, the use of inhibitors is necessary.
Most of the corrosion inhibitors suitable for the protection of zinc are aromatic compounds [7-9]. This class of inhibitors has centers for p-electron and functional groups (such as -C=C-, -OR, -OH, -NR2, -NH2 and -SR). The functional groups provide electrons that facilitate the adsorption of the inhibitor on the metal surface [10-11]. In spite of the broad spectrum of inhibitors synthesised and used for the inhibition of zinc and other metals, the challenge of finding inhibitors that are eco-friendly have prompted several researchers to search for more inhibitors. Most of the eco-friendly inhibitors published in literatures are extracts of naturally occurring compounds [12-17]. Also, studies have been extended to some drugs as corrosion inhibitors for metals, but literature is scanty on the use of erythromycin as an inhibitor for mild steel [18-24].
The objective of our study is to investigate the inhibitive and adsorptive properties of various concentrations of erythromycin for the corrosion of zinc in H2SO4 solutions.
Erythromycin is a broad spectrum antibiotic that is similar to penicillin. It is a microlides and is often use for the treatment of respiratory tract infection. The chemical name of erythromycin is (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-14-ethyl -7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy}-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione.
The molecular formula and molecular mass of erythromycin are C37H67NO13 and 733.93 g/mol, respectively, and its chemical structure is as shown below:
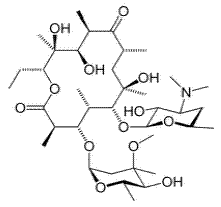
Chemical structure of erythromycin
From the above chemical structure, it can be seen that erythromycin is a large organic molecule that has some functional groups and hetero atoms (O and N) in its structure and is expected to be a good corrosion inhibitor.
Experimental details
Materials
The material used for the study was zinc sheet which was mechanically pressed, cut into different coupons, each of dimension 5x4x0.11 cm. Each coupon was degreased by washing with ethanol, dipped in acetone and allowed to dry in the air before it was preserved in a desiccator. All reagents used for the study were Analar grade and double distilled water was used for their preparation.
The inhibitor (erythromycin) was supplied by LIVEMOORE Pharmaceutical Company, Ikot Ekpene, Akwa Ibom State, Nigeria, and was used without further purification. The concentrations range for the inhibitor (used for the study) was 0.0001 to 0.0005 M.
Gravimetric method
In the weight loss experiment, the pre-cleaned zinc coupon was dipped in 20 mL of the test solution maintained at 303, 313 and 323 K in a thermostated bath. The weight loss was determined by retrieving the coupons at 24 h interval progressively for 168 h (7 days). Prior to measurement, each coupon was washed in 5% chromic acid solution (containing 1% silver nitrate) and rinsed in deionized water. The difference in weight was taken as the weight loss of zinc.
From the weight loss measurements, the inhibition efficiency (%I) of the inhibitor, degree of surface coverage (q) and the corrosion rate (CR) of zinc were calculated using equations 1, 2 and 3, respectively [25]:
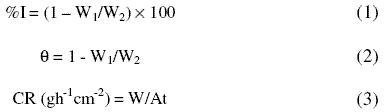
where W1 and W2 are the weight losses (g) for zinc in the presence and absence of the inhibitor in H2SO4 solution, respectively, q is the degree of surface coverage of the inhibitor, A is the area of the zinc coupon (in cm2), t is the period of immersion (in hours) and W is the weight loss of zinc steel after time, t.
Gasometric method
Gasometric methods were carried out at 303 K as described in the literature [26]. From the volume of hydrogen evolved per minute, inhibition efficiencies were calculated using equation 4:
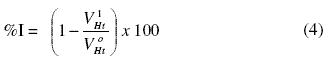
where and are the volumes of H2 gas evolved at time, ‘t’ for inhibited and uninhibited solutions, respectively.
Results and discussion
Effect of concentration of H2SO4/ erythromycin
Figs. 1a to 1c show the variation of weight loss with time for the corrosion of Zn in 0.01 M H2SO4 containing various concentrations of erythromycin at 303, 313 and 323 K. The Figures reveal that the weight loss of zinc decreases with increasing concentration of erythromycin, indicating that the rate of corrosion of zinc in H2SO4 also decreases with increasing concentration of erythromycin. With increasing temperature, weight loss of zinc was found to increase, indicating that the rate of corrosion of zinc in H2SO4 increases with increase in temperature.
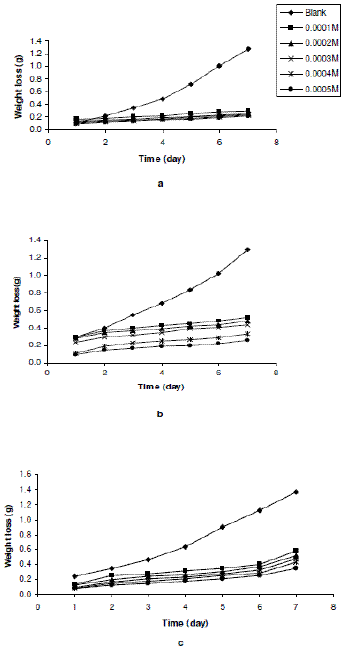
Figure 1. Variation of weight loss of zinc with time for the corrosion of Zn in 0.01 M H2SO4 containing various concentrations of erythromycin at (a) 303, (b) 313 and (c) 323 K.
The variations of weight losses of zinc in 0.02, 0.03 and 0.04 M H2SO4 (containing various concentrations of erythromycin) were also observed to follow patterns similar to those observed for 0.01 M H2SO4 (plots not shown). However, for a given concentration of erythromycin, weight loss of zinc was found to increase with increase in the concentration of H2SO4, indicating that the inhibition efficiency of erythromycin is also affected by the strength of the acid.
The corrosion rates of zinc in various concentrations of H2SO4 and the inhibition efficiencies of various concentrations of erythromycin are presented in Table 1. Table 1 reveals that the corrosion rate of zinc in H2SO4 increases with increase in temperature and with increasing concentration of H2SO4.
Table 1. Corrosion rates (CR) of mild steel in H2SO4 and inhibition efficiencies (%I) of various concentrations of erythromycin for zinc corrosion.
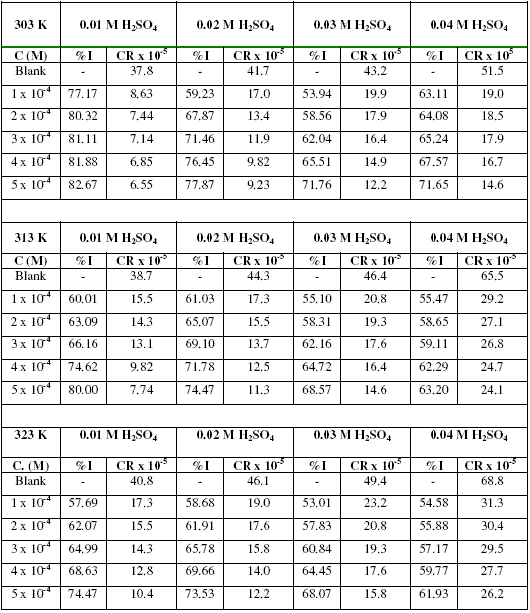
From the results, it is also evident that for a given concentration of H2SO4 and temperature, the inhibition efficiency of erythromycin increases with increasing concentration. Therefore, erythromycin is an adsorption inhibitor for the corrosion of zinc in H2SO4 solutions. From gasometric studies, the inhibition efficiency of 0.0001, 0.0002, 0.0003, 0.0004 and 0.0005 M of erythromycin was 72.34, 82.44, 86.00, 88.20 and 92.22 % respectively. These values are higher than those obtained from weight loss measurements, hence the instantaneous inhibition efficiency of erythromycin is better than its average inhibition efficiency.
Effect of temperature
The Arrhenius equation (eq. 5) was used to study the effect of temperature on the rate of corrosion of zinc in various concentrations of H2SO4 (containing various concentrations of erythromycin as an additive) [27].
![]()
where CR is the corrosion rate of zinc, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant and T is the temperature. Equation 5 can also be written as follows,
![]()
From eq. 5, a plot of logCR versus 1/T should be linear with slope and intercept equal to -Ea/2.303R and logA, respectively. Fig. 2a to 2d present the Arrhenius plots for the corrosion of zinc in 0.01 to 0.04 M H2SO4 (containing various concentrations of erythromycin) respectively.
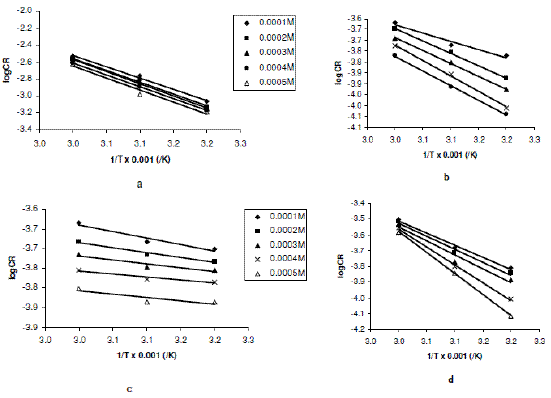
Figure 2. Arrhenius plot for the inhibition of the corrosion of zinc in (a) 0.01 M, (b) 0.02 M, (c) 0.03 M and (d) 0.04 M H2SO4 by various concentrations of erythromycin.
Values of Arrhenius parameters deduced from the plots are presented in Table 2. The results indicate that the activation energies for the blank solutions are lower than those obtained for solutions of H2SO4 containing various concentrations of erythromycin. This also implies that erythromycin retarded the corrosion of zinc in H2SO4 solutions.
Table 2. Some thermodynamic parameters for the inhibition of the corrosion of zinc (in various concentrations of H2SO4) by various concentrations of erythromycin.
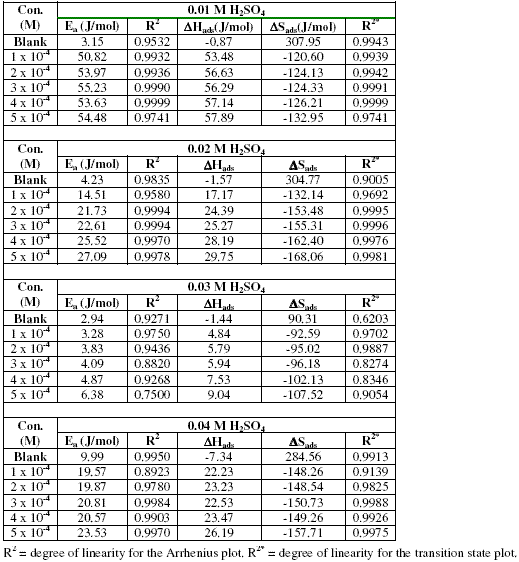
At a given concentration of H2SO4, the activation energies were found to increase with increasing concentration of erythromycin, indicating that there is increasing ease of adsorption of the inhibitor with increasing concentration. We also observed that the activation energies are lower than the threshold value of 80 kJ/mol, hence the adsorption of erythromycin on zinc surface favours the mechanism of physical adsorption [28].
Thermodynamic/adsorption considerations
The transition state equation (eq. 9) was used to calculate some thermodynamic parameters (DHads and DSads) for the adsorption of erythromycin on mild steel surface [29];
![]()
where CR is the corrosion rate of zinc in H2SO4 solutions, R is the gas constant, T is the temperature, N is the Avogadro’s number, h is the Planck constant., DSads is the entropy of adsorption and DHads is the enthalpy of adsorption of the inhibitor on zinc surface. From the logarithm of both sides of equation 7, equation 8 was obtained,
![]()
Plots of log (CR/T) versus 1/T for erythromycin were linear. The slopes and intercepts of the transition state plots (Figs. 3a to 3d) are equal to -DHads/2.303R and (log R/Nh + DSads/2.303R), respectively.
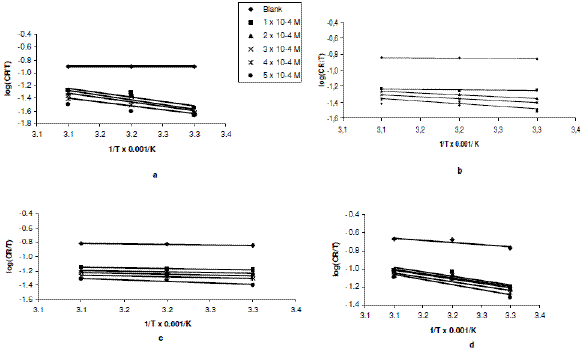
Figure 3. Variation of log(CR/T) with 1/T for the inhibition of zinc corrosion [in (a) 0.01 M, (b) 0.02 M, (c) 0.03 M and (d) 0.04 M H2SO4] by erythromycin.
Values of DHads calculated from the slopes of the plots are positive (Table 2) and ranged from 53.48 to 57.89 kJ/mol, 17.17 to 29.75 kJ/mol, 4.84 to 9.04 and from 22.23 to 26.19 kJ/mol for H2SO4 concentrations of 0.01, 0.02, 0.03 and 0.04 M, respectively. From the calculated values of enthalpy change, it can be seen that the adsorption of erythromycin on zinc surface is exothermic. On the other hand, values of DSads (calculated from the intercept of the transition state plots) are negative. This indicates that there is increasing degree of orderliness and suggests that there is a bigger association of the inhibitor’s molecules rather than dissociation [29].
We noted that there is a progressive increase in the observable thermodynamic parameters (Ea, DSads and DHads) with increasing concentration of erythromycin, which confirms that erythromycin is an adsorption inhibitor for the corrosion of zinc in H2SO4 and explain why the inhibition efficiency of erythromycin increases with increase in concentration.
There was no significant difference between values of DHads and Ea, but both parameters correlated strongly with each other (P>0.05). This can be explained as follows: the Arrhenius equation can be equated with the transition state equation as
![]()
From equation 9, it is evident that the activation energy is related to the enthalpy of adsorption. Therefore, for reactions involving liquids and solids (such as corrosion), D(PV) is negligibly small and since DHad = Ea + D(PV), values of Ea should approximate DHads values, as found in the present study.
The adsorption behaviour of erythromycin for the corrosion of zinc in H2SO4 solutions was studied by fitting data obtained for the degree of surface coverage of the inhibitor (from weight loss measurements) to different adsorption isotherms. From the results obtained, the best model that described the adsorption behaviour of erythromycin is Langmuir adsorption isotherm which can be written as follows [12]:
![]()
where C is the concentration of the inhibitor in the bulk electrolyte, q is the degree of surface coverage and K is the equilibrium constant of adsorption. A plot of log(C/q) versus logC yielded straight lines and is presented in Figs. 4a to 4c.
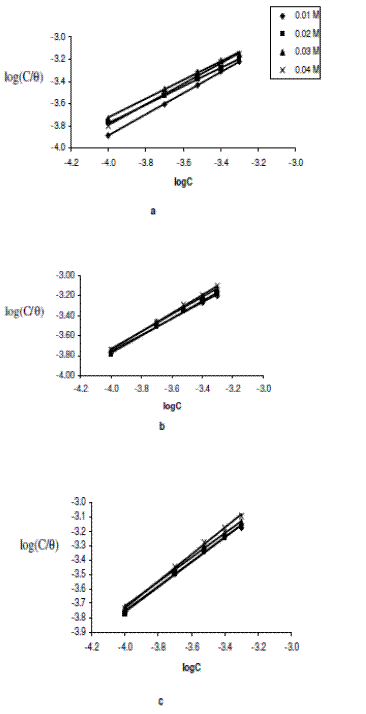
Figure 4. Langmuir isotherm for the adsorption of erythromycin on zinc surface in various concentrations of H2SO4 at (a) 303 K, (b) 313 K and (c) 323 K.
Values of adsorption parameters deduced from the Langmuir isotherm are presented in Table 3. The results obtained show that the slopes and R2 values are very close to unity, indicating strong adherence of the data to the Langmuir adsorption isotherm.
Table 3. Langmuir adsorption parameters for the adsorption of erythromycin on zinc surface in 0.01 - 0.04 M H2SO4 at various temperatures.
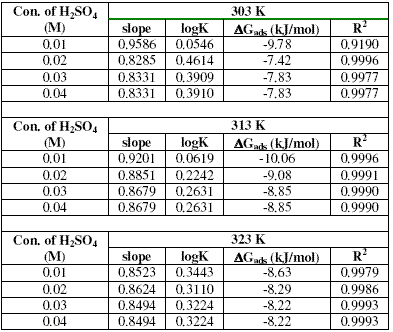
The free energy of adsorption of erythromycin on the surface of zinc is related to the equilibrium constant of adsorption according to equation 11 [26,30]:
![]()
where K is the adsorption equilibrium constant. Table 3 also presents values of DGads calculated from K values (obtained from Langmuir adsorption isotherm). These values are negatively less than the threshold value of -40 kJ/mol, which indicates that the adsorption of erythromycin on the surface of zinc is spontaneous and supports the mechanism of physical adsorption [26].
Conclusion
Erythromycin is an adsorption inhibitor for the corrosion of zinc in H2SO4. The performance of this inhibitor can be optimised by taking advantages of the operating temperature, concentration of the inhibitor or of the acid. Inhibitory action of erythromycin for zinc corrosion can be explained by thermodynamic and adsorption theories.
References
1. S.K. Rajappa, T.V. Venkatesha, B.M. Praveen, Bull. Mater. Sci. 31 (2008) 37.
2. J. Dobryszycki, S. Biallozor, Corrosion Sci. 43 (2001) 1309. 10.1016/S0010-938X(00)00155-4
3. M. Masamitsu, Coating Technol. 34 (1999) 378.
4. A.A. Ahmed, S. Abdel-Hakam, Anti-corrosion Methods Mater. 36 (1989) 4. 10.1108/eb020735
5. K. Aramaki, Corrosion Sci. 44 (2002) 871. 10.1016/S0010-938X(01)00087-7
6. Y.K. Agrawal, J.D. Talati, M.D. Shah, M.N. Desai and N.K. Shah, Corrosion Sci. 46 (2004) 633. 10.1016/S0010-938X(03)00174-4
7. S. Rajendran, J. Jeyasundari, P. Usha, J.A. Selvi, B. Narayanasamy, A.P.P. Regis, P. Rengan, Port. Electrochim. Acta 27 (2009) 153. 10.4152/pea.200902153 [ Links ]
8. S.A. Odoemelam and N.O. Eddy, Mater. Sci. 4 (2008) 1.
9. B. Muller, M. Schubert, G. Kinet, Pigment Resin Technol. 28 (1999) 279. 10.1108/03699429910294328
10. M.A. Quraishi, I. Ahamad, A.K. Singh, S.K. Shukla, B. Lal, V. Singh. Mater. Chem. Phys. 112 (2008) 1035. 10.1016/j.matchemphys.2008.07.011
11. M. Zerfaoui, H. Ouddac, B. Hammouti, S. Kertit, M. Benkaddourb. Prog. Org. Coat. 51 (2004) 134. 10.1016/j.porgcoat.2004.05.005
12. M.A. Bendahou, M.B.E. Benadellah, B.B. Hammouti, Pigment Resin Technol. 35 (2006) 95. 10.1108/03699420610652386
13. E.E. Ebenso, N.O. Eddy and A.O. Odiongenyi, Afri. J. Pure Appl. Chem. 4 (2008) 107.
14. N.O. Eddy, S.A. Odoemelam, A.O. Odiongenyi, J. Appl. Electrochem. 39 (2009) 849. 10.1007/s10800-008-9731-z
15. N.O. Eddy and E.E. Ebenso, African J. Pure Appl. Chem. 2 (2008) 1.
16. M. Abdallah, Port. Electrochim. Acta 22 (2004) 161. 10.4152/pea.200402161 [ Links ]
17. A.O. Odiongeyi, S.A. Odoemelam and N.O. Eddy, Port. Electrochim. Acta 27 (2009) 33. 10.4152/pea.200901033 [ Links ]
18. M. Abdallah, Corrosion Sci. 44 (2002) 717. 10.1016/S0010-938X(01)00100-7
19. N.O. Eddy, S.A. Odoemelam, A.J. Mbaba, Afri. J. Pure Appl. Chem. 2 (2008) 132.
20. N.O. Eddy, U.J. Ibok, E.E. Ebenso, A. El Nemr, E.H. Ashry, J. Molec. Model. 15 (2009) 1085. 10.1007/s00894-009-0472-7.
21. E.E. Ebenso, N.O. Eddy, A.O. Odiongenyi, Port. Electrochim. Acta 27 (2009) 13. 10.4152/pea.200901013 [ Links ]
22. S.A. Odoemelam, E.C. Ogoko, B.I. Ita, N.O. Eddy, Port. Electrochim. Acta 27 (2009) 57. 10.4152/pea.200901057 [ Links ]
23. N.O. Eddy, S.A. Odoemelam, P.A. Ekwumemgbo, Sci. Res. Essay 4 (2009) 33.
24. N.O. Eddy, S.A. Odoemelam, N.W. Akpanudoh, Research J. Pure Appl. Sci. 4 (2009) 1963.
25. H. Ashassi-Sorkhabi, M.R. Majidi, K. Seyyedi, Appl. Surf. Sci. 225 (2004) 176-185. 10.1016/j.apsusc.2003.10.007
26. E.E. Oguzie, B.N. Okolue, E.E. Ebenso, G.N. Onuoha, A.I. Onochukwu, Mater. Chem. Phys. 87 (2004) 394. 10.1016/j.matchemphys.2004.06.003
27. S. Acharya, S. N. Upadhyay, Trans. Indian Inst. Met. 57 (2004) 297.
28. S.T. Arab, A.M. Al-Turkustani, Port. Electrochim. Acta 24 (2006) 53. 10.4152/pea.200601053 [ Links ]
29. M.M. Attar, J.D. Scantlebury, JCSE 8 (2005) 97.
30. G.K. Gomma, Mater. Chem. Phys. 55 (1998) 241. 10.1016/S0254-0584(98)00155-2
Received 30 May 2009; accepted 5 November 2009
* Corresponding author: nabukeddy@yahoo.com














