Introduction
The skin, the largest organ in the human body1, performs mechanical, immunological, and antimicrobial protection functions and participates in thermal regulation and sensory perception. It also has a rich network of hormone receptors and is therefore sensitive to hormonal changes, which can result in a wide variety of skin manifestations that represent a diagnostic challenge for general practitioners, endocrinologists, and dermatologists2.
Endocrine disorders can lead to significant changes in the skin and phanera and may serve as clinical markers of these underlying conditions. Cutaneous changes associated with these disorders can range from very mild to very severe, vary from non-specific to specific to the underlying disease, and may appear early or late in the course of these diseases3.
By understanding the complex interrelationships between the skin and the endocrine system, in several situations, health-care professionals can identify underlying endocrine disorders early and provide appropriate and effective treatment, which can significantly improve the quality of life and life-span of these patients4. This is particularly important in situations where these cutaneous alterations represent paraneoplastic manifestations of aberrant hormonal production by tumor cells5.
Acromegaly
Acromegaly is a rare and chronic disease that results from the overproduction of growth hormone (GH) after the closure of the bone epiphyses6. It is mainly caused by pituitary adenomas, which produce excess GH and is characterized by an increase in blood levels of GH and insulin-like growth factor type 1 (IGF-1)7.
The characteristic signs and symptoms of acromegaly are related to increased levels of GH and IGF-1, which results in the overgrowth of soft tissues and organs, with subsequent clinical manifestations, such as enlargement of the extremities (hands and feet), tongue, jaw, facial changes (protruding forehead and enlarged nose), joint pain, sleep disturbances, voice changes, among others.
Dermatological manifestations
The excess GH can cause an increase in skin thickness, which is due to the increase of dermal collagen, and not to the expansion of the epidermis due to the proliferation and maturation of keratinocytes8. In addition, these patients may have swollen skin due to the accumulation of glycosaminoglycans in the dermis, which is more prominent on the face, hands, and feet9. Skin tags, which are usually related to insulin resistance and diabetes mellitus (DM), are present in most individuals affected by acromegaly. It is unclear if its appearance is due to excess GH and IGF-1 or if it is a consequence of another metabolic dysregulation10. Excessive sweating is found in 50% to 88% of patients, due to the trophic effect of GH on the cutaneous sweat glands, as well as growth of the periglandular nerves11.
Hypothyroidism
Hypothyroidism with low amounts of thyroid hormones results in a lower metabolic rate12. The most common causes are Hashimoto’s thyroiditis, treatment of hyperthyroidism, surgical removal of the thyroid, or the use of certain medications, such as methimazole, propylthiouracil, and amiodarone13. If not properly treated, it can lead to weight gain, fatigue, constipation, depression, infertility, heart disease, and decreased cognitive function. Treatment is done with thyroid hormone replacement14.
Dermatological manifestations
Dry, cold, and rough skin, due to decreased blood flow and secretion from the acinar glands, in response to a drop in thyroid hormones, are the most common manifestations, resulting in flaking, itching, and hypersensitive skin14. Changes in skin pigmentation are also observed. Pallor may occur due to decreased cutaneous blood supply, a yellowish coloration is usually due to increased serum carotene levels, and also cutaneous hyperpigmentation can be observed when hypothyroidism is associated with primary adrenal insufficiency15.
There may also be weakening of the nails, which become brittle, fragile and prone to flaking. This is due to hypothermia caused by hypothyroidism, resulting from a decrease in metabolic rate, which leads to a compensatory secondary vasoconstriction. Vasoconstriction decreases blood flow, with a deficit in the supply of nutrients and oxygen to skin structures, causing slow growth and brittle nails16.
The hair may also become coarse, brittle, dry, develop slow growth, and eventually lead to alopecia17.
In addition, myxedema may occur in the lower limbs, a soft and painless edema, giving the skin a thick and flaccid appearance, especially on the face, periorbital region, hands, and feet which results from the infiltration of the skin with glycosaminoglycans with associated water retention18.
Hyperthyroidism
The most common causes of hyperthyroidism are Graves’ disease19, and hyperfunctioning thyroid nodules20. It can lead to a number of complications, including unintentional weight loss, tachycardia, palpitations, anxiety, tremors, muscle weakness, irritability, insomnia, heat intolerance, exophthalmos, cardiovascular, bone, and fertility changes21.
Dermatological manifestations
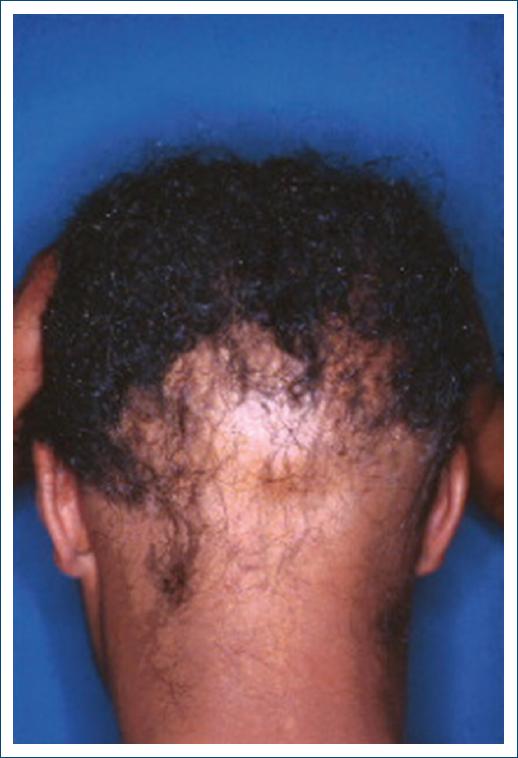
Among the most common dermatological manifestations are warm, moist, and smooth skin. Heat is due to increased cutaneous blood flow and peripheral vasodilation, resulting from increased metabolism and may be accompanied by hyperhidrosis22. The skin may appear thin, soft, and fragile, becoming more susceptible to injuries and bruises, because of the decrease in the keratin layer. Diffuse or generalized alopecia (Fig. 1) may also be found23.
Nails can present with onycholysis, also called Plummer’s nails, which comprises the separation of the nail from the nail bed, and onychodystrophy, which involves changes in the shape and texture of the nails. Some patients may develop cutaneous hyperpigmentation, especially in the areas exposed to the sun. The mechanism is related to cortisol deficiency, due to its accelerated degradation that occurs in these patients, which leads to an increased production of pro-opiomelanocortin (POMC), a prohormone that is subsequently cleaved into adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormone (MSH). Elevated serum levels of MSH stimulate increased melanin synthesis, which leads to hyperpigmentation24.
If the cause of hyperthyroidism is Graves’ disease, periorbital edema and protrusion of the ocular globe may also be found. This is due to the enlargement of the eye muscles, caused by inflammation due to the accumulation of inflammatory cells and proliferation of fibroblasts in the interstitial tissue. Thus, this muscle enlargement ends up impairing the venous drainage of the orbital contents, causing periorbital and conjunctival edema25.
Other autoimmune diseases affecting the skin can also observed more frequently in these patients, namely, vitiligo and chronic urticaria26.
Polycystic ovary syndrome (PCOS)
It affects women of reproductive age and is characterized by hormonal changes that generally lead to the formation of multiple cysts in the ovaries, hyperandrogenism, weight gain, and fertility disorders27. Although not yet fully understood, the causes of polycystic ovary syndrome PCOS are associated with genetic and hormonal factors, associated with insulin resistance28. Complications such as menstrual irregularities, infertility, obesity, type 2 DM, cardiovascular disease, and mood disorders can occur. Diagnosis is based on clinical criteria, laboratory tests, and imaging29.
Dermatological manifestations
Several alterations can be found in affected patients, such as hirsutism29 and acne, resulting from hyperandrogenism30,31. This is due to the fact that androgens are the main hormonal regulators of hair growth and sebum production and secretion. In women with PCOS, excess androgen production by the ovaries increases serum levels of testosterone and dihydrotestosterone which interact with androgen receptors in hair follicles and promote differentiation from fleece hair (small, thin, and poorly pigmented) into terminal hair (thick, long, and pigmented)32 and enhance the activity of sebaceous glands. Hirsutism occurs mainly in the genital area and can progress along the medium line, groins, lumbar region, face, chin, intermammary region, and around the nipples33. Acne in PCOS affects especially the face, but also the neck, upper trunk, and back regions.
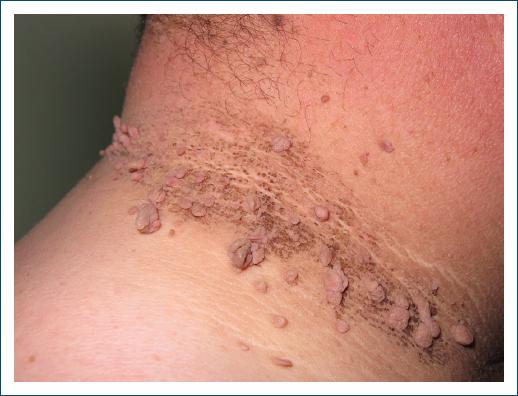
Acanthosis nigricans (Fig. 2), characterized by dark, thick patches of skin, can also be found, especially in areas of body folds, such as the neck, armpits, and groin. It is strongly related to insulin resistance present in PCOS, with excessive production of insulin and IGF-1, which leads to increased stimulation of keratinocytes and fibroblasts29.
Addison’s disease
Addison’s disease, also known as primary adrenal insufficiency, is a rare condition34 resulting mainly from autoimmune destruction of these glands or infections, such as tuberculosis35. Addison’s disease can lead to serious complications such as fluid and electrolyte imbalance, hypotension, chronic fatigue, weight loss, dizziness, and, in extreme cases, adrenal crisis or Addisonian crisis, a potentially life-threatening medical emergency36. Treatment involves replacing deficient adrenal hormones such as cortisol and aldosterone37.
Dermatological manifestations
Cutaneous hyperpigmentation (Fig. 3) is the most characteristic finding, occurring in about 90% of patients35, which is a consequence of cortisol deficiency and increased production of POMC and MSH by the hypophysis. Elevated MSH results in increased melanin synthesis in basal melanocytes (Fig. 4), causing hyperpigmentation36. This hyperpigmentation is most common in sun-exposed areas and in skin folds, such as joints and scars. The color varies from light brown to dark brown and can be more intense on mucous membranes, such as gums, tongue, cheeks, and lips38.
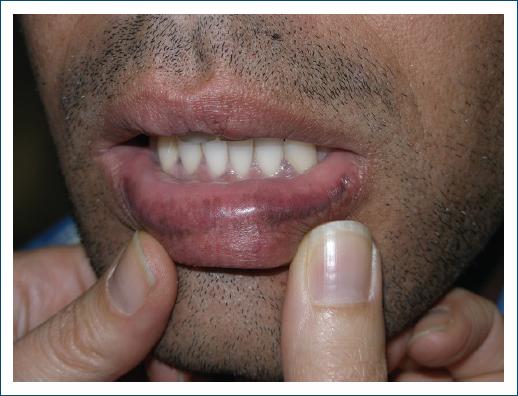
Figure 3 Hyperpigmentation on the lips of a patient with Addison’s disease (source: University Hospital of Coimbra).
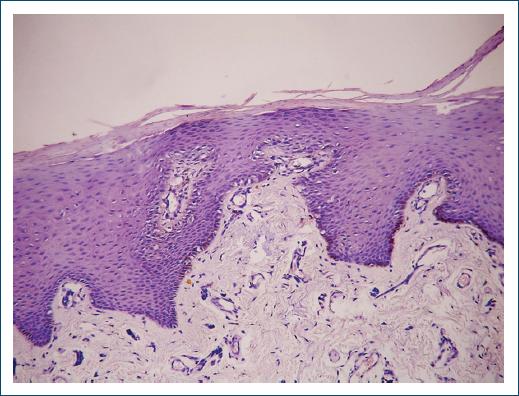
Figure 4 Histopathology (H and E ×40) of basal melanocytes of a patient with Addison’s disease (source: University Hospital of Coimbra).
Vitiligo, in unequal areas and often bilateral, may be present in 10% to 20% of patients with Addison’s disease with autoimmune etiology, due to the autoimmune aggression of melanocytes39.
Cushing’s syndrome
It is a condition that results from excessive exposure to high levels of cortisol40, which plays an important role in regulating metabolism, the immune system, and stress41. The causes of Cushing’s syndrome can be varied, including the use of exogenous corticosteroids, tumors in the adrenal glands or pituitary gland, and, in rare cases, cortisol-producing tumors in other organs42.
The complications of this syndrome can be serious and compromise several systems of the body. In addition to weight gain, patients may have redistribution of body fat, with accumulation in the abdomen and face, with a decrease in fat tissue in arms and legs40. Other signs and symptoms may include muscle weakness, increased blood pressure, emotional changes, menstrual irregularities in women, and sexual dysfunction in men. In addition, these patients may have osteoporosis and DM41.
Dermatological manifestations
One of the most prevalent manifestations is the presence of stretch marks in various regions of the body, such as the abdomen, thighs, arms, and breasts42 (Fig. 5), which are reddish or purplish in the acute phase and become thinner and whitish over time43. Their presence is related to the reduction in skin elasticity caused by excess cortisol, which decreases the production of collagen and elastin, important components for skin integrity. High levels of cortisol can also inhibit cell regeneration and proper skin healing. When the skin is thin, it is more vulnerable to injury, and may present frequent bruising44.
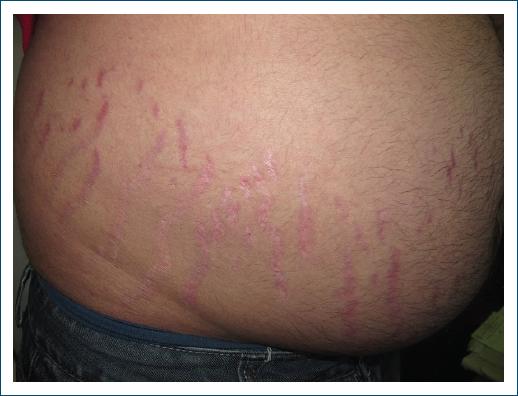
Figure 5 Stretch marks in a patient with Cushing’s syndrome (source: University Hospital of Coimbra).
Another common skin manifestation when the cause is a pituitary tumor producing high levels of ACTH is hyperpigmentation, which can develop in sun-exposed areas, as well as in skin folds, armpits, groin, and neck. The skin in these areas may become darker, and the coloration may vary from light brown to dark brown45.
Cushing’s syndrome can also lead to the development of oily skin and monomorphic papular acne, due to the hyperstimulation of the sebaceous glands caused by cortisol. Facial erythema and flushing can also be found, especially on the cheeks and nose, a condition known as facial erythema46.
Hypopituitarism
Hypopituitarism is a rare condition that can occur due to damage or dysfunction in the pituitary gland itself or in the surrounding structures, due to the presence of pituitary tumors, traumatic injuries, infections, inflammations, and complications after cranial surgeries or radiation therapy or post-partum (Sheehan syndrome)47.
The clinical manifestations of this disease can be diverse, such as excessive fatigue, unintentional weight loss, muscle weakness, menstrual disorders in women, sexual dysfunction in men, changes in growth and development in children, hypotension, stress intolerance, and metabolic disorders, among others48.
Dermatological manifestations
Dermatologic findings depend on which glands are compromised and which hormones are insufficient. In case of insufficiency in the production of thyroid-stimulating hormone the skin may become dry, cold, and rough, due to the resulting hypothyroidism, with all the clinical manifestations of this disease4.
In addition, the decrease in the production of gonadotropins follicle-stimulating hormone, and luteinizing hormone results in secondary hypogonadism in men and women, with a consequent decrease in body hair and also alopecia, since sex hormones are essential for the maintenance of hair and hair follicles44.
DM
DM is characterized by altered insulin secretion and varying degrees of peripheral insulin resistance, causing hyperglycemia and abnormal glycosylation of several molecules, namely, hemoglobin (HbA1C) whose levels are accurately related to the glycemic control49. Initial symptoms are related to hyperglycemia and include polydipsia, polyphagia, polyuria, and blurred vision. Late complications include vascular disease, peripheral neuropathy, kidney disease, and predisposition to infections50. Most complications can be postponed or prevented with adequate glycemic control51.
There are two main types of DM: type 1 and type 2 DM.
Type 1 DM is an autoimmune disease in which the immune system attacks and destroys insulin-producing pancreatic beta cells, leading to absolute insulin deficiency49. Type 2 DM, on the other hand, is characterized by insulin resistance and/or deficiency50. While type 1 DM usually develops at younger ages and cannot be prevented, type 2 DM generally occurs in people older than 45 years and is strongly associated with obesity, physical inactivity, and family history, and can often be prevented or delayed with lifestyle changes49.
Dermatological manifestations
Diabetic dermopathy is present in about 40% of patients and is often related to some DM-related chronic complication, such as kidney disease, retinopathy, or neuropathy52. The disease tends to appear in the lower extremities and in older men and can result from minor trauma53. It is characterized by painless, and rarely itching, atrophic and hyperpigmented macules, located on the anterior region of the legs. They are oval or round, depressed, shiny, irregularly contoured, sometimes bilateral but with asymmetrical distribution54.
Skin infections occur in 20-50% of patients with DM55, particularly in those with type 2 DM. Overall, they are associated with poor glycemic control. Several factors favor the onset of infections in these patients, mainly those with chronic vascular or neurological complications. Alterations in immune response are frequently found, especially reduced neutrophil chemotaxis and phagocytosis, with increased adhesion between epithelial cells and pathogens (such as Candida albicans in the oral and vaginal mucosa and Escherichia coli in the urinary tract)56.
Acanthosis nigricans often precedes the diagnosis of hyperglycemia, is characterized by dark, thick patches of skin, and is most often found in areas of body folds, such as the neck, armpits, and groin. It is strongly related to insulin resistance, which promotes the excessive production of IGF-1, which leads to hyperstimulation of keratinocytes and fibroblasts. The result is the appearance of hyperkeratosis and epidermal papillomatosis, which can be observed in histopathological analysis. Hyperkeratosis is mainly responsible for the clinical manifestation28.
Vulvovaginal candidiasis is a prevalent disorder found in women with DM (Fig. 6), especially in those with poor glycemic control, and is a frequent cause of vulvar pruritus56. It is clinically manifested by erythema of the genital area (with or without fissures) and satellite pustules. It is sometimes accompanied by whitish vaginal discharge. Its pathophysiology is related to the immune deficit of individuals with DM since they present inhibition of neutrophil activity, which increases the proliferation, adhesion, and virulence of this microorganism57.
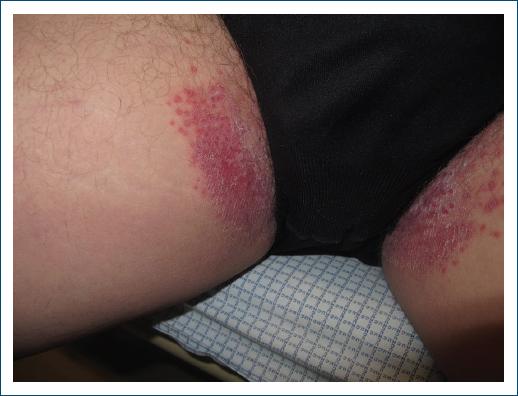
Figure 6 Groin candidiasis in a type 1 diabetic patient with poor glycemic control (source: University Hospital of Coimbra).
Diabetic foot is the most devastating of DM-related complication. It is usually due to a combination of vasculopathy, neuropathy, and mechanical trauma, which occurs in 15-25% of all people with DM, and in about a quarter of these patients can lead to the onset of infections and osteomyelitis, which can lead to amputation of the affected limb58. It is generally due to an unnoticed trauma to cutaneous areas with neuropathy and calluses, such as on the soles of the feet and tips of toes. The healing process is generally seriously compromised, due to hyperglycemia, impaired chemotaxis, cell proliferation, and migration59. In addition, the increase in pro-inflammatory chemokines hinders wound healing, leading to the appearance of plantar ulcers. The presence of neuropathy and vasculopathy in these feet can cause muscle atrophy and bone deformation, with important changes in bone architecture, causing the collapse of the arch of the foot, a condition called Charcot foot.
Chronic pruritus is a common manifestation of DM, occurring in 3-49% of patients, significantly affecting their quality of life60. Diabetic polyneuropathy with sweating dysfunction due to sympathetic nervous system impairment may play a role in the pathogenesis of pruritus in these patients61.
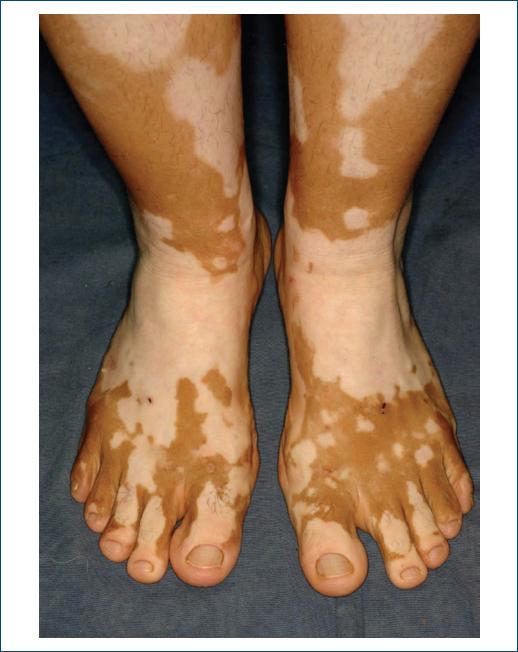
Vitiligo (Fig. 7), is found in 2-10% of patients with type 1 DM62, occurring spontaneously with a progressive course. Its pathophysiology is related to autoimmune mechanisms, an intrinsic characteristic of this type of DM63.
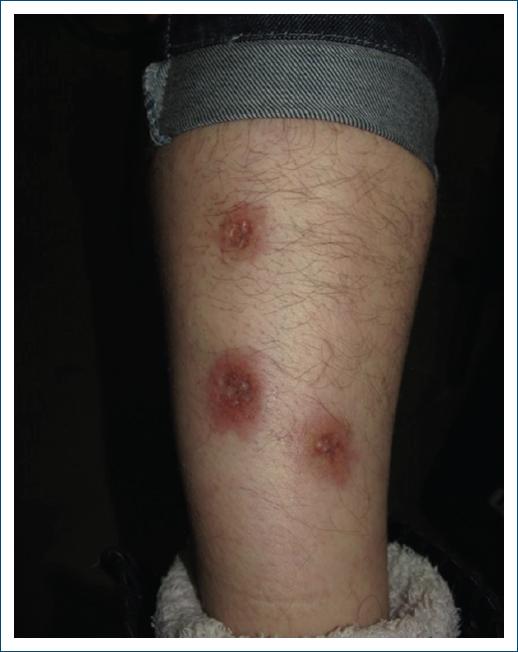
Necrobiosis lipoidica (Fig. 8) affects 0.3-1.2% of all patients with DM, with a higher prevalence in women than in men, and is commonly located in the ventral area of the legs, often with symmetrical distribution64. It usually begins with erythematous papules, which slowly evolve into a brownish-yellow plaque with atrophic center and telangiectasia. In up to 35% of patients, lesions may ulcerate and present secondary bacterial infections. The pathophysiological mechanisms are still unknown, but the histopathology of the lesions shows degeneration of dermal collagen with a palisading granulomatous inflammatory infiltrate under an atrophic epidermis65. Glycemic control has no significant effect on the evolution of these lesions, and no study has convincingly proven the efficacy of the various types of drugs used in the treatment of necrobiosis lipoidica, namely, topical or intralesional corticosteroids injected into the lesion edges, and also ultraviolet A56.
Conclusion
The cutaneous manifestations associated with endocrine disorders are varied and may serve as valuable clinical markers for these underlying conditions. Understanding the complex interactions between the skin and the endocrine system is crucial for early identification of endocrine disorders and providing appropriate treatment. The examples covered in this narrative review article highlight how dermatological examination can play a key role in the detection and management of endocrine diseases, significantly improving the quantity and quality of life of patients. Therefore, collaboration between dermatologists, endocrinologists, and general practitioners is essential for effective diagnosis and treatment of these complex and often underdiagnosed conditions.