Introduction
Anaphylaxis is a severe and potentially life-threatening allergic reaction that can be triggered by several substances and medications. Glucocorticoids, commonly prescribed in allergic reactions for their potent anti-inflammatory properties, are generally well-tolerated and considered safe. However, in rare instances, glucocorticoids themselves can become the unsuspecting culprits behind anaphylactic reactions1. This paper presents a rare clinical case of anaphylaxis induced by glucocorticoid administration.
Case report
Our case focuses on a 34-year-old woman, pregnant, with a history of allergic rhinitis treated with antihistamines as needed. She had no documented history of drug allergies.
During pregnancy, she was prescribed hydrocortisone cream for an irritant contact dermatitis, with resolution of the dermatosis.
At 38 weeks of gestation, the patient experienced a vaginal delivery complicated by an unintentional dural puncture for epidural analgesia. The baby was born healthy.
Three days after labor, she received intravenous hydrocortisone for postpartum pain management. During administration, within minutes, the patient developed severe dyspnea, with decreased oxygen saturation, dysphonia, and generalized urticaria. She was brought to the emergency room and was treated with intramuscular adrenaline and antihistamines with clinical improvement. Serum tryptase concentration measured 1 h after emergency treatment was 1.7 ng/mL (normal range 0-11.4 ng/mL).
The patient was referred to the imunoallergology department for further study. Skin prick tests, intradermal skin tests, and challenge tests were performed.
Prick tests for aeroallergens were positive for Dermatophagoides pteronyssinus (10 mm), Dermatophagoides farinae (10 mm), Lepidoglyphus destructor (10 mm), cat dander (10 mm), dog dander (6 mm), grass pollen (6 mm), and Artemisia vulgaris (10 mm).
Prick tests for methylprednisolone succinate 10 mg/ml and hydrocortisone succinate 10 mg/mL and 100 mg/ml were positive (4 mm, 3 mm, and 4 mm, respectively), whereas those for dexamethasone sodium phosphate 4 mg/ml, budesonide 0.25 mg/ml, and latex were negative (Fig. 1).
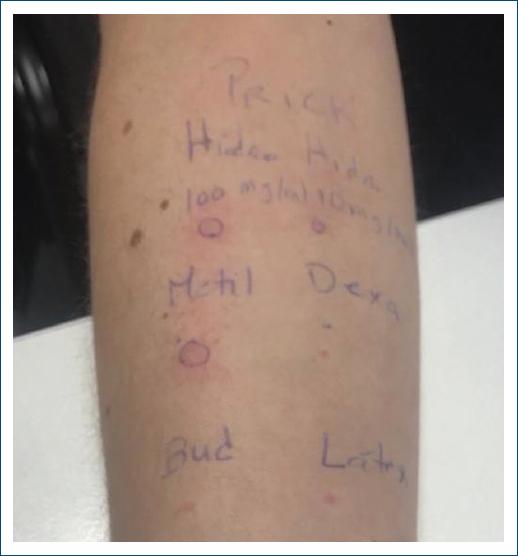
Figure 1 Prick tests positive for methylprednisolone succinate 10 mg/ml and hydrocortisone succinate 10 mg/mL and 100 mg/ml.
Intradermal skin tests for dexamethasone sodium phosphate 0.004 mg/ml and budesonide 0.0025 mg/ml were negative.
A diagnosis of anaphylactic reaction to group A corticosteroids was made. The patient was instructed to completely avoid hydrocortisone, methylprednisolone, and prednisolone.
In challenge testing with nasal budesonide and oral dexamethasone, the patient showed no reaction, making these a safe alternative corticosteroid.
Discussion
Glucocorticoids, such as prednisone and hydrocortisone, are widely used in the management of various inflammatory and immunological disorders, including anaphylaxis. Despite their extensive use, reports of anaphylaxis resulting directly from glucocorticoids remain infrequent, making this clinical case of importance. Anaphylaxis secondary to glucocorticoids that contain succinate esters is reported to have a prevalence of 0.3% and about 0.1% of parenteral administrations2,3.
The mechanisms underlying glucocorticoid hypersensitivity reactions are not completely understood. These reactions may occur due to an abnormal immune response to glucocorticoids or their excipients. Genetic predisposition, previous exposure to glucocorticoids, and underlying immune disorders can play a role.
Glucocorticoid hypersensitivity reactions can manifest in various ways and the severity of symptoms can range from mild to severe. They can be divided into two categories: immediate reactions, occurring within 1 h of drug administration, and non-immediate reactions, manifesting more than an hour after drug administration. The latter group is the most common, with allergic contact dermatitis following topical corticosteroids application being the most frequently reported4.
Signs and symptoms of immediate hypersensitivity to glucocorticoids include pruritus, rash, hives, angioedema, shortness of breath, coughing, nausea, vomiting, abdominal pain, fever, and, in rare cases, anaphylactic shock. Hypersensitivity reactions have been reported following intravenous, intramuscular, oral, intra-articular, epidural, and topical administration5-8. In this case, the patient developed anaphylaxis within minutes of intravenous administration.
Diagnosing glucocorticoid hypersensitivity reactions can be challenging. Prick, patch, intradermal, and challenge testing with glucocorticoid preparations can be performed to confirm hypersensitivity, identify potential cross-reactivity with other medications, and provide long-term management strategies9. Prick testing is suggested initially, and, if negative, intradermal testing is then performed, and, in many cases, a challenge testing is also needed7,9. Prick and intradermal test results are considered positive when the wheal produced is at least 3 mm larger than that elicited by normal saline control9. In this case, we performed prick tests, which confirmed the diagnosis, and intradermal testing followed by challenge testing to identify safe alternatives.
Based on stereochemistry, corticosteroids are classified into five groups: A, B, C, D1, and D2. Substances from the same group are thought to cross-react, but a patient sensitized to one or a group of glucocorticoids does not have to avoid all types of glucocorticoids. Challenge testing is the best way to select alternatives that are safe1,9. In this case, the patient was sensitized to group A corticosteroids but tolerated budesonide and dexamethasone.
Rapid recognition and appropriate management of anaphylaxis are crucial to prevent potentially fatal outcomes, and accurate identification of the causative agent is pivotal to prevent subsequent exposure.
It is important to stay vigilant when prescribing medication, even in patients with no prior history of adverse reactions. The potential for idiosyncratic responses to any medication, including those considered relatively safe, must be acknowledged and carefully evaluated.
In conclusion, this case report highlights the importance of recognizing anaphylaxis induced by glucocorticoids and aims to contribute to the growing body of literature on immediate hypersensitivity reactions to glucocorticoids.














