INTRODUCTION
Diabetes mellitus (DM) is one of the most serious health problems worldwide, reaching epidemic proportions, mainly due to the rapidly rising global prevalence of type 2 DM.1,2Chronic kidney disease (CKD) is a common complication of DM, which further contributes to cardiovascular risk and mortality.3-6 Nowadays, it is commonly accepted that diabetic kidney disease (DKD), traditionally called diabetic nephropathy (DN), is a clinical and pathologically heterogeneous disease, affecting approximately 20 to 40% of diabetics, type 1 or 2, and accounting for roughly 45% of patients on renal replacement therapy.7
It no longer indicates a specific pathological phenotype and is mainly a clinical diagnosis, based upon the presence of albuminuria (> 30mg/dl), and/or decreased estimated glomerular filtration rate (eGFR), or both, in a diabetic patient.8,9
The etiology of DKD is multifactorial. The magnitude and duration of hyperglycemia and hypertension are of utmost significance, in addition to genetic and environmental factors.10 Other determinants that increase the probability of DKD or accelerate its development are glomerular hyperfiltration, smoking, obesity, sedentarism, dyslipidemia,
proteinuria and high fat and carbohydrates diet.11 Concerning pathophysiology, DKD results from a metabolic and hemodynamic impairment, promoting inflammation, endothelial dysfunction, oxidative stress, fibrosis and glomerular hyperfiltration. All these events lead to kidney lesions and potentiate cardiovascular events.12
Although in type 1 DM, with five or more years duration, DN is likely the cause of DKD, particularly if associated with diabetic retinopathy (DR), in type 2 DM, this correlation can range widely, as the prevalence of concomitant non-diabetic kidney disease (NDKD) increases.13-15In these cases, only a kidney biopsy (KBx) can accurately settle if DKD is due to DN. Furthermore, albuminuria is no longer required to make a clinical diagnosis of DKD. A substantial minority of diabetic patients and decreased eGFR, with less than 30 mg/g of albuminuria, have histopathological findings consistent with DKD. Moreover, the regression from moderately increased albuminuria to normoalbuminuria is common in type 1 DM and, although at lower rates, can also occur in type 2 DM.16,17
On the other hand, a presumptive diagnosis of DKD should be avoided if there are features present that raise suspicion of na alternative diagnosis for the kidney disease, namely, severely elevated albuminuria (> 300 mg/g) within five years of onset of type 1 DM, or many years before type 2 DM diagnosis; red blood cell casts or dysmorphic red blood cells in the urinary sediment; the presence of a systemic disease with renal involvement (e.g. systemic lupus erythematous) or a sudden or atypical increase albuminuria or eGFR decrease. In these patients, a KBx should usually be performed18-19
The histological findings consistent with DN are closely dependente on each health center’s biopsy policy.20,21 KBx indication can range from a “restricted” policy to only patients with a suspected alternative diagnosis or an “unrestricted” approach to any diabetic patient who presents with severe albuminuria, low eGFR, or hematuria. One study, as an example, examined biopsies from patients where an alternative diagnosis was suspected. Of those, 29% had classic diabetic glomerulopathy alone; conversely, with an “unrestricted” approach, 33% had an alternate diagnosis besides DKD, and another one-third showed only NDKD.22 The most common diagnosis of NDKD reported in recente literature were acute tubular necrosis (ATN), immune-mediated glomerular diseases, hypertensive nephrosclerosis and focal segmental glomerulosclerosis (FGSF).23
Patients with type 1 DM predominantly develop classical diabetic glomerulopathy, whereas type 2 diabetics, particularly those without albuminuria, may have a myriad of pathological findings.24 The earliest recognized pathological abnormality is the thickening of the glomerular basement membrane, which can occur as early as two years after the diagnosis in type 1 DM. It is followed by mesangial expansion and nodular (Kimmelstiel-Wilson nodules) or diffuse glomerular sclerosis.25 Arteriolar hyalinosis and arteriosclerosis of larger vessels are common, likely representing the combined effect of hyperglycaemia and hypertension. Tubulointerstitial fibrosis anticipates the progression to advanced CKD and end-stage kidney disease (ESKD). As already mentioned, the histological abnormalities in type 2 DM are more heterogeneous, with a more severe vascular and tubulointerstitial disease, most likely translating the distinct phenotypes of these patients.26
Several studies have tried to establish clinical hints to differentiate DKD from NDKD. The most cited include DR, the duration of DM, proteinuria, and hematuria. Unfortunately, none has attained enough sensitivity or specificity to dismiss a KBx.27,28
In the present study, the authors aimed to study clinical or laboratory factors predictive of DKD or NDKD in diabetic patients, establishing, whenever possible, correlations with histological features.
SUBJECTS AND METHODS
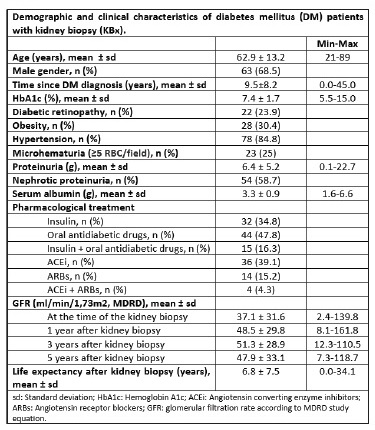
We conducted a retrospective study in diabetic patients submitted to a native kidney biopsy (KBx) at the Nephrology Department of Centro Hospitalar e Universitário de Coimbra (CHUC), between January 1990 and December 2020. Electronic medical records were reviewed to collect demographic and clinical data concerning age, gender, time since DM diagnosis, time of death, coexistent comorbidities (DR, hypertension, obesity), and their pharmacological treatment, namely use of angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), insulin or oral antidiabetic drugs. Laboratory data gathered included serum creatinine, serum albumin, glycated hemoglobin (HbA1c) levels, urinary sediment, spot urine protein/creatinine ratio (UPCR), and 24-hour collection proteinuria. For follow-up purposes, we considered serum creatinine levels at the time of KBx and at one, three, and five years after the procedure. eGFR was estimated based on the Modification of Diet in Renal Disease (MDRD) equation study.
The indications for KBx in our cohort included the following criteria: proteinuria in the nephrotic range (>3.5 g/day) or CKD in the absence of diabetic retinopathy; nephrotic proteinuria or CKD within five years of onset of DM; isolated nephrotic proteinuria; nephrotic syndrome (proteinuria >3.5 g/day associated to edema and/or serum albumin <3.5 g/dL, with or without hematuria); unexplained microscopic hematúria (presence of five or more red blood cells per high power field in urine analysis), acute kidney injury (AKI) (increase in serum creatinine by ≥0.3 mg/dL within 48 h or ≥1.5 times baseline within seven days) and an unexplained rapidly worsening renal function in patients previously stable.
We reviewed histopathological reports of those patients and considered diffuse or nodular mesangial expansion, thickening of glomerular basement membranes, and nodular glomerulosclerosis as markers of DN and organized these findings according to the classification outlined by Tervaert et al.25 Tubulointerstitial lesions, interstitial inflammation, arteriolar hyalinosis, and diffuse linear staining for IgG along tubular basement membranes and glomerular capillaries were also considered supportive diagnostic features. Based on KBx findings, patients were categorized as isolated DN, isolated NDKD, or DKD (NDKD superimposed on DN).
Statistical analysis was performed using and SPSS® software (version 20). Normal distributed continuous variables were presented as mean ± standard deviation (sd). Categorical data are shown as numbers and frequencies (percent, %). We used T-student, chisquared, or Fisher’s exact tests to compare differences between groups whenever pertinent. Univariable and multivariable logistic regression models were used to explore risk factors for NDKD. Survival analysis was estimated by the Kaplan-Meier method. The diferences between DKD and NDKD groups were assessed using the log-rank test. We considered a two-sided α of less than 0.05 as statistically significant.
RESULTS AND DISCUSSION
We analysed data from 96 diabetic patients who underwent a KBx at our hospital between 1990 and 2020. Four patients were excluded due to an insufficient histological sample to establish a diagnosis. The mean age of our final population was 62.9 ± 13.2 years, ranging between 21 and 89 years. KBx was performed, on average, 9.5 ± 8.2
years after the diagnosis of DM. Only 71.7% of the patients had previous ophthalmological screening examinations, of whom 33.3% had DR, including all the five type 1 DM patients (5.2%). Hypertension and obesity were known in 84.8% and 30.4%, respectively. Table I sumarizes demographic and clinical data for the whole sample.
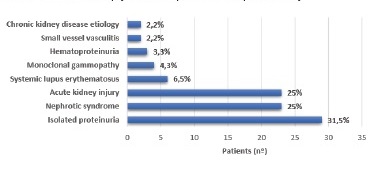
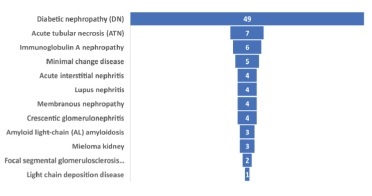
A quarter of our patients was biopsied for nephrotic syndrome (n=23, 25%) or AKI (n=23, 25%), with the most frequent indication for renal biopsy being proteinuria (n=29, 31.5%) (Figure 1). Based on histopathological findings, nearly half of them had isolated DN (n=49, 53.3%), and 14 (15.2%) had DN superimposed on NDKD, comprising a total of 63 patients (68.5%) with DKD. Using the Tervaert pathological classification, nodular sclerosis and advanced diabetic glomerulosclerosis (classes III and IV) were more frequently observed in the isolated DN group (85.7%). In comparison, mild to severe mesangial expansion (class II) was more prevalent in DN superimposed on NDKD patients (68.3%). Twenty-nine patients (31.5%) were considered to have NDKD. All type 1 diabetic patients were included in the DN group. Besides DN, ATN was the main histological final diagnosis (n=7, 7.6%), closely followed by IgA nephropathy (n=6, 6.5%) and minimal change disease (n=5, 5.4%). All the pathological diagnoses are listed in Figure 2.
When KBx were performed, patients with NDKD were less likely to need insulin therapy (24.1% vs. 62.5%, p=0.002), had lower albumin levels (2.9 vs. 3.4 mg/dL, p=0.03), and a higher prevalence of microhematuria (41.9% vs. 10.4%, p=0.001). We found the latter to be na independent predictor of NDKD; despite ATN accounting for 7.6%
(n=7) of this group, the majority of patients with microhematuria had glomerulopathies.
For all the cohorts, the mean eGFR was 37.1 ± 31.6 ml/min/1.73m2 at the time of KBx and 47.9 ml ± 33.1 ml/min/1,73m2 at the fifth year of follow-up. This improvement of eGFR over time is clearly related to the significant percentage of patients with AKI in our population.
However, we found a significant negative association between the rate of decline of eGFR and the presence of histological DKD, with NDKD patients evincing a greater loss of renal function five years after KBx (R2 -0.339, p=0.004). Likewise, patients presenting with acute deterioration of renal function were significantly more prevalente among the NDKD group (60.9% vs. 30.4%, p=0.01).
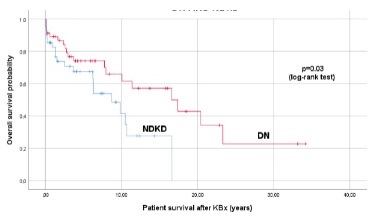
When analysing survival data, we also found that NDKD patients had a significantly lower global life expectancy compared to DKD patients (8.4 ± 1.2 vs. 16.6 ± 2.4) (p=0.03) (Figure 3).
DISCUSSION
The role of a KBx in DM patients remains controversial and the criteria to perform it lacks consensus. Besides the potential use for DN-related investigational purposes, this invasive procedure in diabetic patients seems mainly helpful in excluding NDKD. In accordance with previously published series, our patients were submitted to KBx when the clinical and laboratory findings raised the suspicion of conditions other than DKD. Severe or rapid onset proteinuria, especially if less than five years from the diagnosis of type 1 DM or in the absence of concomitant DR, was the most common indication for requiring a KBx in our sample. The coexistence of other systemic diseases with wellknown renal involvement, the evidence of hematuria or AKI were also frequent criteria. This heterogeneity of indications in daily medical practice highly explains the assorted prevalence of NDKD in clinical studies, with values ranging from 6.5% to 94%.29 In our series, DN was excluded in almost a third of the patients studied, all with type 2 DM, bringing a global prevalence of NDKD of 31.5%.
Several diagnoses had been reported in diabetic patients with NDKD, with significant heterogeneity in the histological patterns pictured among different populations. A systematic analysis by Fiorentino and colleagues concluded that IgA nephropathy was the most common renal pathology overall, mainly in Asia, with a higher frequency of
FGSF described in European studies.18 In our analysis, ATN was na important histological finding, the leading diagnosis after classic DN. Similarly, an American study by Sharma et al. concluded that ATN was the most common NDKD, found alone or alongside DN lesions in 28.4% of those patients.22 These results highlight the acknowledged susceptibility of diabetic patients to ischemia and tubulointerstitial damage and are of clinical relevance, since ATN is associated with a higher risk of progression towards ESKD, especially in the setting of the reduced renal reserve associated with DM.22,32
Certain factors, such as microscopic hematuria (especially with active urinary sediment), severe or sudden-onset proteinuria, the absence of diabetic retinopathy, a short duration of DM, low HbA1c levels, and acute deterioration of renal function have been described as clinical predictors of NDKD.29,30 Accordingly, we found microhematuria and kidney function impairment, either acutely or progressive, to be more common among the NDKD group.
Whereas the need for insulin therapy has been frequently associated with DN, its absence was considered a strong predictor of NDKD among 80 Croatian diabetic patients studied by Horvatic et al. 29,31
In our analysis, insulin independence was also a distinctive marker of NDKD, probably reflecting a more accurate metabolic control in this subgroup of patients where DM was not the predominant pathology.
Finally, low albumin levels prevailed among histopathological NDKD, which might be related to the significant proportion of patients biopsied for albumin-wasting events, such as in nephrotic syndrome cases or inflammatory conditions, as those found in AKI patients.
The presence and extent of the pathological lesions assessed by KBx in diabetic patients have proved its prognostic value, with isolated or mixed forms of DKD meaning higher chances of progression to ESKD.26 In our cohort, NDKD was associated with a significantly lower life expectancy when compared to DKD, a different result from the one recently reported by Bermejo et al., who inferred that DN was an independent risk factor for mortality.27 Our results further disagree with other data published reporting better renal and overall survival of NDKD when compared to DKD.28,29 The severity of comorbidities diagnosed among our patients, some of them with systemic involvement, may partially explain the poorest survival in the NDKD cohort.
There are many limitations in our study. The small number of patients and a monocentric, retrospective design with a relatively short follow-up period may explain the paucity of clinically significant predictors of NDKD found among our cohort. Similar to other studies, there was also a significant selection bias related to the KBx criteria, raising the risk of overestimating the actual prevalence of NDKD in patients with DM. However, to our knowledge, this is the first study characterizing patterns of renal disease among the Portuguese diabetic population, as others focused only on patients with clinical suspicion of NDRD,32 acquiring strength in the urge of gathering multicentric data and consensual approaches in order to provide the best renal care to our patients.
In conclusion, KBx remains the gold standard approach for diagnosis, treatment decisions and outcome prediction in patients with kidney diseases. However, currently, there are no standardized criteria or consensus about mandatory indications nor clinical usefulness in patients with DM. Therefore, the decision to perform a kidney biopsy in these patients should be based on clinical judgment and health centers’ policies. In our cohort, a significant proportion of patients yielded an NDKD and, therefore, was potentially worthy of receiving targeted therapies to improve kidney function. The evidence of na acute deterioration of renal function, microhematuria, low albumin levels and absence of insulin therapy were independent predictors of NDKD, suggesting their potential use for determining the histological assessment in those patients. However, the decision to perform a KBx in DM should be individualized and based on careful clinical judgment to promote our patients’ best management, ensuring early diagnosis, particularly of NDKD, and timely treatment, thus avoiding irreversible sequelae, and improving renal and overall prognosis.