Introduction
Central venous catheters (CVC) devices have been used in hematology oncology patients who require high dose chemotherapy treatment (CT), transfusion support, and blood sampling for an extended period of time (Zakhour, Chaftari, Raad, 2016). Large osmolarity spectrum drugs, multiple infusions, and perfusion volumes related to treatment and secondary complications (e.g., septicemia) lead to a higher continuous CVC use ratio (Joint Commission Resources, 2012).
Catheter-related occlusion due to mechanical obstruction and catheter-related infection are the most important complications in the management of CVCs (Baskin et al, 2009; Callister et al, 2015. Catheter-related occlusion can occur from two different sources: thrombotic and nonthrombotic (Baskin et al, 2009; Cesaro et al, 2004). A clot is considered the most common cause of thrombotic occlusion. Occlusion (partial and complete) should be considered when the capacity to withdraw blood is compromised and the ability to flush fluids is lost (Smith et al, 2017). Nonthrombotic causes such as catheter pinch-off, precipitation of drugs, and catheter migration can result in the inability to aspirate blood as well (Smith et al, 2017). Catheter-related occlusions usually are reported in the literature in the second and third week after CVC placement (Napalkov et al, 2013).
Thrombotic occlusions could be associated with treatment-related factors such as bolus chemotherapy infusions, antigenic or platinum therapy (irritant and exfoliant drugs), chest radiation therapy, erythrocyte stimulating agents and parenteral nutrition (Kitchens, Konkle & Kessler, 2013). Moore and colleagues performed in 2008 a large retrospective analysis of all patients treated with cisplatin-based chemotherapy for any type of malignancy at Memorial Sloan-Kettering Cancer Center. The study showed that 169 (18.1%) patients reported thrombosis events during the treatment or within 4 weeks after the last dose, suggesting the necessity of studies of cancer patients undergoing cisplatin-based regimes (Moore et al, 2011). However, research into this issue remains scarce.
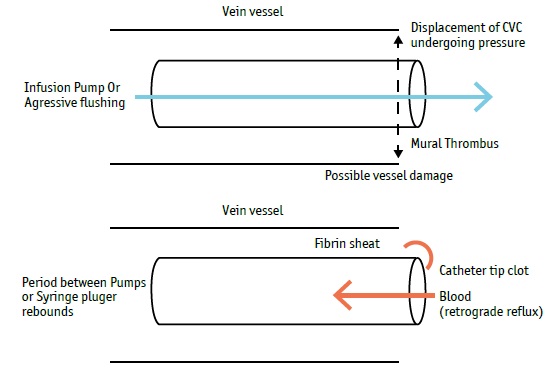
The continuous infusion pump flow rate and aggressive flushing increase the risk of endothelial damage associated with the continued vessel wall contact by the CVC tip (Gunawansa, Sudusinghe & Wijayaratne, 2018). Moreover, it could increase the risk of a filling defect near the catheter tip or retrograde flow along the external surface of the catheter leading to extrinsic fibrin sheath formation (which causes up to 43% of catheter dysfunction), (Beard, Gaines & Loftus, 2013). Blood withdrawal and flushing procedures (e.g., ineffective positive pressure and push-pause techniques) could increase the accumulation risk of blood deposits in the catheter lumen. In these cases, the fibrin deposit can progress to an intrinsic thrombosis, such as intraluminal thrombus or fibrin tail formation on the catheter tip (Hadaway, 2005).
The aim of this study is to evaluate the occlusion events associated with a new platinum-based regimen undergoing salvage with continuous infusion pump flow rates.
Methods
Selection and description of participants
A single-center prospective comparative study was performed, including all consecutive cases of occlusion events associated with a new platinum-based regimen (DHAP) in hematology oncology patients using a CVC since August 2018 to September 2019 (Phase 1), and October 2019 to September 2020 (Phase 2) at the Onco-Hematology Department of the Portuguese Institute of Oncology (Porto).
Patients older than 18 years old, with the new platinum-based regimen (DHAP) and CVC were included.
Phase and Platinum-based regime (DHAP) technical information
Phase 1: Observational period using a platinum-based regime (DHAP) undergoing continuous infusion pump rate ≥ 200 mL/hr.
Phase 2: Intervention period using a platinum-based regime (DHAP) undergoing continuous infusion pump rate ≤ 200 mL/hr. To reduce the continuous infusion pump rate, volume redistribution between perfusions was performed.
DHAP: Dexamethasone (po or IV, day 1-4), High-dose Ara-C-cytarabine (IV infusion over 2 hrs, day 2, every 12 hrs) and Platinol (IV infusion over 24 hrs, day 1). Infusion therapy volume including IV hydration therapy and CT in the first 36 hrs ≥ 9000 mL.
Occlusion definitions
Occlusion was considered when the capacity to withdraw blood was compromised and the ability to flush fluids was lost (Smith et al, 2017). Partial occlusion (inability to aspirate blood, but ability to infuse fluids through the catheter lumen) and complete occlusion (inability to aspirate blood and infuse fluids through the catheter lumen) were considered. Catheter lock was considered when the solution was injected into the catheter lumen dead space until it was to be accessed again (Smith et al, 2017).
Technical Management CVC Hospital Policy
The management of CVCs follows the CDC (2011) guidelines. Double lumen Hickman® type catheters (Vygon®) are used (7 French, lumen No.1=0.6 and lumen No.2=1.0). Control chest x-ray was always performed after CVC insertion.
Specific CVC flush and catheter care includes the use of SASH technique ( Smith et al, 2017), catheter lock with 10 mL normal saline solution flush and anticoagulant (Fibrilin®), positive pressure technique (Hadaway, 2005), volume of syringe used ≥ 10 mL, neutral split-septum needleless connector (Bionecteur®), and push-pause flushing (± 2.5 mL pulses using ≥ 10 mL normal saline solution).
Flushing frequency was reporting in periods ≤ 72 hrs in hospital admissions and periods ≤ 30 days after hospital discharge.
Alteplase protocol was performed by syringe or stopcock method declotting when complete occlusions were reported.
Data Analysis
Data analysis is conducted using IBM SPSS Statistics for Windows (SPSS Inc., Version 24.0). A continuous variable was reported by median and range. Categorical variables were reported as frequency and percentages. Normality tests reported a sample without normal distribution, considering that, hypothesis tests were analyzed by non-parametric tests. Relative Risk was performed by confidence interval of 95%. A p value of ≤ 0.05 was determined to be statistically significant.
Results
A total number of 43 occlusion events were identified in the study period (phase 1, n=28 vs phase 2, n=15). The DHAP regime occlusion events were reported in 32.5% (n=14) cases. Overall, 39 DHAP regimens were reported, with no identified significant DHAP regimen distribution differences between phases (phase 1, n= 21 vs phase 2, n=18, p>0.05). The occlusion risk associated with the infusion pump rates between phases undergoing DHAP regime was higher in phase 1 (phase 1, n=11 vs phase 2, n=3, RR 3.313 [1.010 to 13.863], ≤ 0.05).
Flushing protocol was performed a median of 17 (6 to 22) days after discharge. In 78.6% (n=11) cases the occlusion event was reported after 30 days of CVC-life (median 43 days, 12 to 126). The CVC was removed in 64.3% (n=9) of occlusion events. Restoration of CVC patency undergoing alteplase protocol was observed in 35.7% (n=5) cases. A complete occlusion in the CVC 0.6 mm lumen was observed in each case, and complete occlusion of the CVC 1.0 mm lumen at the same time as occlusion of the CVC 0.6mm lumen on 2 occasions. When aspiration clot (n=5) was identified, the CVC was always removed. No aspiration clot was observed in phase 2.
Discussion
The infusate nature (exfoliant and irritant drugs), patient characteristics (hematology oncology patients), device characteristics (multi-lumen CVC), or proposed duration of venous access (long-term CVC) are important factors to vascular access indication (Zakhour, Chaftari & Raad, 2016; Joint Commission Resources, 2012; Baskin et al, 2009; Cesaro et al, 2004; Napalkov et al, 2013; Chopra et al, 2015). In the DHAP regime all these factors are observed. In oncology hematology patients, partial occlusions could influence the blood sampling procedures; as PICC occlusion rates are higher in this population, the Hickman CVC could be considered a safer option for CT in these cases (Lim et al, 2013).
Theoretical rationale approach
In the first 36 hrs of the DHAP regime, the salvage infusion therapy volume, multi-lumen CVC use (multiple continuous perfusions), blood transfusions, monoclonal antibody administration, and use of platinum-based regime could increase the risk of generating high turbulence, pressure and reflux in the CVC tip, and possible vessel damage as well.
Since the beginning of DHAP administration, an increasing number of complete occlusions were identified. In some cases, complete occlusions were observed during blood sampling, as the CVC was functional (blood withdrawal of 3 mL/3s, (Cummings- Winfield & Mushani, 2008)) until a clot was aspirated into the CVC lumen. In other cases, complete occlusions were observed between infusion pumps or in the final stage of bolus drug administration as well. The negative pressure applied between infusion pumps and bolus drug administration could suggest a higher risk of retrograde flow along the external and internal lumen surface. (Figure 1)
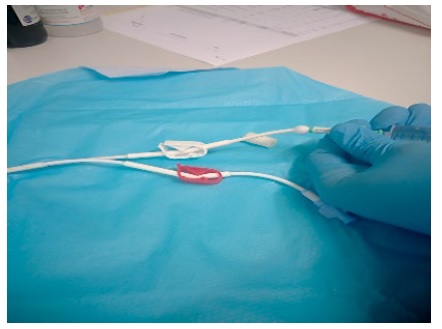
When the CVC patency was not restored and the CVC was removed, it was tested with a 10 mL syringe saline solution administration being a “bubble” formation in the hub observed. (Figure 2) The CVC was sectioned and a clot (±3 cm) in the proximal section (CVC-line bifurcation) was reported.
Aspiration Clot: Infusion pumps and Aggressive flushing
Changes in infusion pressure can lead to thrombus formation resulting from reflux (Hadaway, 2005). In our study, an aspiration clot was never observed in the first DHAP regime, reinforcing the idea that the extraluminal clot was produced in this phase, then aspirated and identified in subsequent CT phases. After the first DHAP regime, negative pressure applied through the CVC lumen during blood withdrawal could remove the external CVC surface fibrin tail and aspirate it into the lumen. The clot was aspirated through the CVC lumen until the Hickman® bifurcation (Figure 3), as the difference between lumen sizes is an important factor to position the clot in this region. Indeed, the narrower lumen increased the reflux distance observed, which could explain the higher frequency of complete occlusion observed in the 0.6 mm CVC lumen.
When infusion pump flow rates are increased, the negative pressure applied through the CVC lumen between pumps could aspirate the clot through the catheter tip into the lumen (Hadaway, 2005).The inappropriate drug bolus administration by aggressive flushing through the administration system could produce the same effect as well (syringe plunger rebounds and draws blood back), (Hadaway, 2005). In both cases, an aspiration clot resulting in catheter tip occlusion could be observed. (Figure 4)