Introduction
Breast cancer is the malignancy with the highest incidence and mortality between women worldwide1. These tumors may complicate with metastasis, including rare cutaneous metastasis. In fact, after melanoma, breast cancer has the highest incidence rate of cutaneous metastasis among the solid malignancies2. Usually, cutaneous metastasis presents as a nodular disease, even though inflammatory, cicatricial, and bullous presentations are possible2. Zosteriform distribution of cutaneous secondary neoplasia is a rare clinical presentation3. In this paper, we report a case of a florid pigmented cutaneous metastasis of breast carcinoma with a zosteriform clinical presentation.
Clinical case
A 77-year-old woman, born in Manaus, state of Amazonas, Brazil, with a previous diagnosis of invasive ductal carcinoma of the left breast in 2015, underwent a total mastectomy following chemotherapy in the same year. In 2022, she was referred to the dermatology clinic due to pigmented lesions present for 2 years, initially on the bed of the mastectomy, which progressively spread to the entire left hemithorax and ipsilateral back. During this period, the diagnosis of shingles (herpes zoster) was suggested, but the patient did not have any clinical improvement despite antiviral therapy.
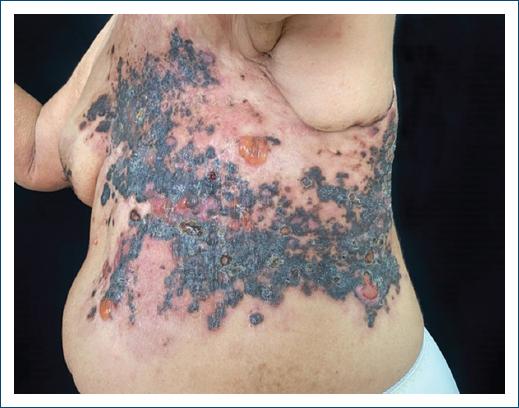
Dermatological examination revealed extensive infiltrative blackish papules and plaques, with a hard consistency. There were some flaccid blisters with serohematic content. The lesion was following a zosteriform distribution in the left thoracodorsal region, more specifically dermatomes T2 through T8 (Figs. 1 and 2). No lymphadenopathies were detected at clinical examination.
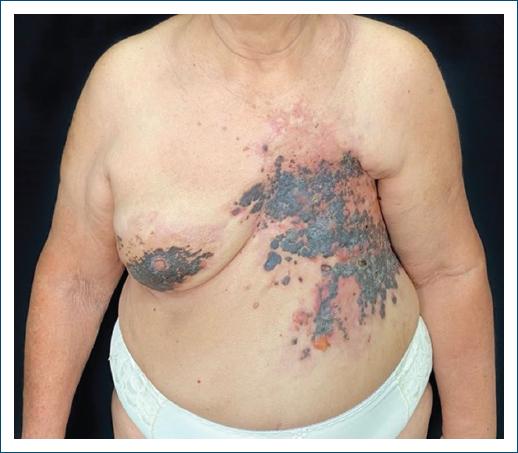
Figure 1 Extensive infiltrative blackish papules and plaques, with increased consistency, and some flaccid blisters with serohematic content.
The main dermoscopy findings were peripheral dots, globules and some radiating striae, with irregular distribution, over an amorphous area, in addition to areas with the appearance of a blue-white veil, suggestive of a melanocytic lesion (Fig. 3). Thorax, abdomen, and pelvis CT scan (CT-TAP) and bone scintigraphy did not show any signs of metastatic disease.
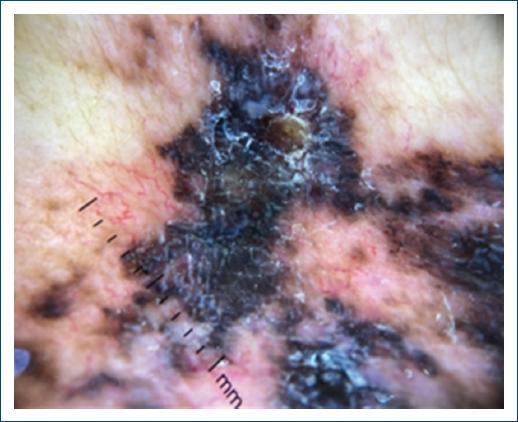
Figure 3 Dermoscopy: peripheral dots, globules, and some radiating striae in irregular distribution over an amorphous area and areas with a blue-white veil.
Histopathology showed atypical epithelioid neoplastic cells, arranged in cords in the superficial and deep dermis, reaching the hypodermis. The presence of intracytoplasmic melanin pigment was observed in the atypical cells located in the superficial dermis (Fig. 4A and B). The lesion was positive for AE1/AE3, ER and GATA-3. Melanocytic markers (Melan-A and S100) were negative. Based on these findings, the diagnosis of skin infiltration by invasive carcinoma of mammary origin associated with melanocytic colonization was established.
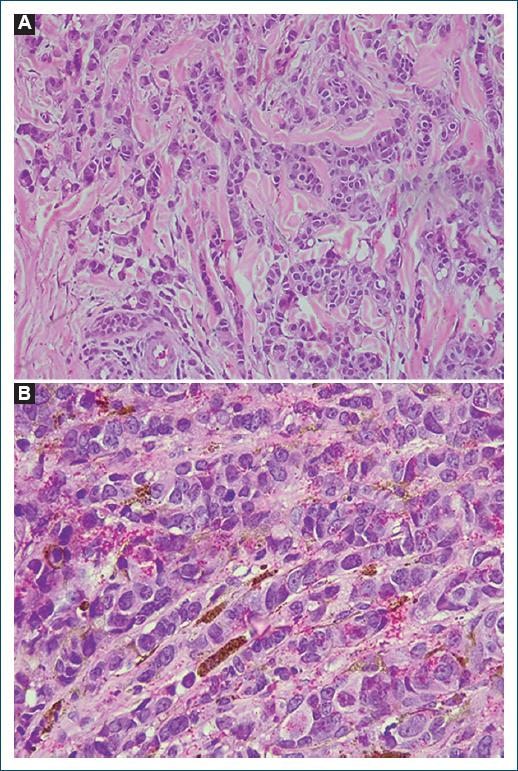
Figure 4 A: neoplastic cells with rounded, hypertrophic, hyperchromatic nuclei, with evident nucleoli, forming small nests and cords in desmoplastic stroma. H/E. Magnification = ×200. B: intracytoplasmatic and stromal melanin deposits. H/E. Magnification = ×400.
At the moment, the patient is undergoing follow-up at the oncology outpatient clinic, undergoing chemotherapy.
Discussion
First described by Azzopardi and Eusebi (1997), pigmented cutaneous metastases are rare and atypical manifestations of breast carcinoma. The lesions can mimic melanoma lesions and are generally found in the thoracoabdominal region, especially on the site of the scar from a previous mastectomy3,4. Initially, Azzopardi and Eusebi (1997) proposed that pigmentation would be related to the rupture of the basement membrane secondary to tumor invasion, determining pigment spillage, dermal melanophages, and migration of epidermal melanocytes3. Secretion of growth factors by malignant cells would be related with the migration and survival of these dendritic melanocytes5.
However, cases without pagetoid involvement demand other explanations to justify the presence of melanocytes in the metastatic tumor stroma5. There is evidence that multipotent dermal stem cells are capable of differentiating into melanocytes. Melanoblasts residing in the bulb region of hair follicles can also differentiate into dermal melanocytes6. Therefore, melanocytic colonization of cutaneous metastases from breast carcinoma can also occur by melanocytes of non-epidermal origin.
The zosteriform distribution of cutaneous metastasis of breast carcinoma is rare, with few cases reported in literature, the first being described in 19337,8. A review of the literature of zosteriform cutaneous metastasis showed that, among 15 total cases, four were from patients with primary breast malignancy, being the most frequent primary tumor associated with this manifestation9. The pathophysiological explanation to the zosteriform disposition of these metastases is not well understood, but there is some hypothesis of possible mechanisms: lymphatic dissemination of locoregional tumors, köebnerization of previous sites of varicella-zoster, iatrogenic seeding of malignant cells, and neural dissemination through dorsal ganglia8.
Successful treatment of the primary tumor is often sufficient for regression of the skin lesions. However, a direct approach to the cutaneous lesion can help reduce tumor burden, improve quality of life, and increase functionality. Treatment modalities include direct surgical excision, radiotherapy, laser or radiofrequency ablation, cryotherapy, and alpha-interferon injections10.
Unusual forms of cutaneous metastases from breast carcinoma, as described in this case report, become real diagnostic challenges. For such cases, clinical suspicion, associated with histopathological and immunohistochemical examinations, is essential. With an accurate diagnosis, appropriate treatment can be carried out more immediately, guaranteeing a significant improvement in the quality of life of our patients.
Conclusion
Breast cancer’s rare cutaneous metastasis, such as the pigmented zosteriform type described here, poses diagnostic and treatment challenges. Understanding these manifestations is critical for accurate diagnosis and timely intervention. Multidisciplinary collaboration and advanced diagnostic tools play pivotal roles in optimizing patient care. Further research is needed to refine management strategies for such atypical presentations.