Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência & Tecnologia dos Materiais
versão impressa ISSN 0870-8312
C.Tecn. Mat. v.21 n.3-4 Lisboa jul. 2009
Electrochemical impedance applied to the corrosion behavior of dental amalgams in synthetic physiological fluids
V. A. Alves(1)*, D. G. Souza(2), R. Q. Reis(2), L. A. da Silva(1) and A. Rossi(1)
(1) Curso de Licenciatura em Química, Instituto de Educação. Universidade Federal do Triângulo Mineiro – UFTM. Av. Getúlio Guaritá, 159, Abadia. 38025-440 Uberaba, Minas Gerais, Brazil
(2) Departamento de Farmácia, Faculdade de Ciências Biológicas e da Saúde. Universidade Federal dos Vales do Jequitinhonha e Mucuri - UFVJM. Rua da Glória, 187, Centro. 39100-000 Diamantina, Minas Gerais, Brazil
* valeria.alves@quimica.uftm.edu.br
ABSTRACT: The dental amalgam is a metallic alloy commonly utilized as a restorative material. In this study, Electrochemical Impedance Spectroscopy, polarisation curves and Mott-Schottky analysis were used to evaluate the physical characteristics of the passive film formed on amalgam surface; X-Ray and UV spectrophotometric analyses were also used to determine the alloy phases and possible soluble corrosion products, respectively. This study was done with Duxalloy® and Tytin® Plus samples in four different electrolytes: Phosphate Buffer Saline, Hank Solution, Artificial Saliva and NaCl 0.9%. In general, the longer the immersion time, the smaller the resistance, possibly due to film breakage; Duxalloy® presented, at the end of 168 h of immersion, a higher resistance when compared to Tytin® Plus. X-Ray analysis showed differences between the two studied brands. UV spectrophotometric analysis demonstrated a small absorption band at ~276 nm. These films kept their protective properties after 168 h of immersion
Keywords: Dental amalgams, Electrochemical Impedance Spectroscopy, Corrosion, Mott-Schottky, Polarisation Curves.
RESUMO: O amálgama dentário é uma liga metálica amplamente utilizada em restaurações dentárias. Neste trabalho, foram usadas a Espectroscopia de Impedância Eletroquímica, curvas de polarização e análises Mott-Schottky para avaliar as características físico-químicas do filme passivo formado sobre a superfície das amostras de amálgama. As técnicas de difração de raios X e a espectrofotometria no ultravioleta também foram utilizadas para determinar as microfases presentes, bem como a formação de possíveis produtos de corrosão que sejam solúveis, respectivamente. Os estudos foram realizados com as amostras de amálgama das marcas Duxalloy® and Tytin® Plus, imersas em quatro diferentes tipos de eletrólitos: Solução salina de tampão fosfato, solução de Hank, saliva artificial e soro fisiológico (NaCl 0,9%). Em geral, quanto maior o tempo de imersão, menor a resistência do biomaterial, devido, possivelmente, à uma ruptura do filme passivo; as amostras da marca Duxalloy® apresentaram, após 168 h de imersão, uma maior resistência quando comparada com aquela das amostras da marca Tytin® Plus. As análises por difração de raios X mostraram diferenças entre as duas marcas estudadas. As análises por espectrofotometria no ultravioleta mostraram uma banda de absorção em ~ 276 nm. Os filmes passives mantiveram suas propriedades protetoras após 168 h de imersão.
Palavras chave: Amálgamas dentários, Espectroscopia de Impedância Eletroquímica, Corrosão, Mott-Schottky, Curvas de polarização.
1. INTRODUCTION
In recent years, many studies have been done in order to investigate the corrosion resistance of dental amalgams under different conditions due to concerns with its toxicity.
Dental amalgam is a metallic alloy formed by the reaction of mercury with a powder alloy containing silver (40-70%), tin (15-30%), copper (10-30%) and sometimes, a small percentage of zinc. The composition and form of the powder alloy influence the microphase structure of the amalgam. Depending on the production technique and shape of the powder alloy particles, the amalgam can be classified as lathe cut (irregular particles), spherical (particles produced by spraying) or blend (mixed spherical and irregular alloy particles). The smaller the particle the shorter the setting time and the less the corrosion [1].
Another important aspect of the structure of dental amalgams is on the microphases themselves. The physical properties of the amalgam depend on the relative percentage of the principal microphases, γ-Ag3Sn, γ1-Ag2Hg3 and γ2-Sn7Hg. The weakest component is the γ2 phase, which is the most subject to corrosion. The high-copper alloys do not have any γ2-Sn7Hg in the final set mass. The η-Cu6Sn5 phase formed in high-copper alloys is not an interconnected phase like the γ2 phase, and is believed to have a better corrosion resistance. The γ-Ag3Sn phase was considered to be electrochemically stable in the oral environment [2]. The amalgamation reactions may be summarized as:
γ-Ag3Sn + Ag-Cu + Hg à γ1-Ag2Hg3 + γ2-Sn7 Hg + γ-Ag3Sn + Ag-Cu (1)
followed by
γ2-Sn7Hg + Ag-Cu à η-Cu6Sn5 + γ1-Ag2Hg3 (2)
In the oral cavity, a dental amalgam filling, like any other object, interacts with a complex and variable environment. In this interaction it is subjected to chemical, biological, mechanical, electrical and thermal forces. As a result, changes take place on restorations surface, composition, structure and properties which may affect the efficacy of the filling. At the same time, the oral environment is also affected with the release of metal ions by electrochemical reaction with the oral fluids [3].
During the last 10 years, many papers have been published on the corrosion of dental amalgam and the recording of polarization curves is one of the most widely used electrochemical methods for this kind of study. With non-stationary techniques, e.g. electrochemical impedance, it is possible to obtain more information of the corrosion phenomena [4]. The Electrochemical Impedance Spectroscopy, EIS, can be particularly powerful for helping in the interpretation of the electrochemical behavior of complex interfacial processes and complex materials, and has already been applied to the investigation and analysis of the processes occurring at the surface of the amalgam [5].
Mott-Schottky analyses for amalgams are scarce in the literature. This type of analysis is a complement to EIS and polarisation curves and it allows a more profound view of the semiconductive properties of the passive films formed on the surface of amalgams. This passive film formation is more evident in some electrolytes than others, reflecting its dependence on the electrolyte composition.
Another type of analysis is X-Ray Diffraction, XRD, which is very important to analyze and confirm the crystalline phases present in the dental amalgams.
In this work, EIS, polarisation curves, Mott-Schottky analysis, UV spectrophotometry and X-Ray Diffraction were done in order to evaluate the resistance to corrosion of dental amalgams characterizing the semiconductive properties of the passive films formed on their surfaces. These analyses were done in four different solutions: Phosphate Buffer Saline, PBS, Hank Solution, Artificial Saliva and NaCl 0.9%. UV spectrophotometric measurements were done after the impedance studies to detect possible soluble corrosion products in the electrolytes.
2. EXPERIMENTAL
2.1 Electrodes and instrumentation
Duxalloy® (Metalms Ltda, Brazil) and Tytin® Plus (S. S. White Artigos Dentários Ltda, Brazil) powder alloys were used to make six samples, three of each brand. The powder alloy composition of Duxalloy® is silver 45%, copper 24% and tin 31%; the powder alloy Tytin® Plus has a high amount of copper and is zinc-free. These alloys were mixed with liquid mercury K-Dent® (Quimidrol Comércio Ltda, Brazil) in a mechanical vibrator Mixalloy® (Indústrias Reunidas Rhos Ltda, Brazil) for 50 seconds. The mercury-to-alloy ratio was approximately 50%. The amalgam was cast into a cylinder-shaped plastic piece of diameter ~ 0.7 cm and length ~ 2 cm.
The plastic pieces were not totally filled so that a copper wire was fixed behind the amalgam with a little portion of the mercury-alloy mixture (inside the plastic pieces) and covered with a layer of Araldite® (Brascola LTDA, Brazil) epoxy-resin adhesive. They were left to dry for 24 h. The prepared sample had one face with amalgam and one with dried epoxy-resin adhesive. Finally, a conductivity test was done to confirm if current was passing through the amalgam to the copper wire. A smooth mirror-like finish was obtained on the amalgam surface polishing with emery paper 600 grit. The exposed electrode area was 0.385 cm2.
A three electrode system was mounted with a working electrode (the amalgam sample), an Ag/AgCl as reference electrode and a Pt platinised (geometric area 1 cm2) auxiliary electrode.
All the electrochemical assays were carried out using a Gamry® Potentiostat, Series G750, monitored by Gamry® Instruments Framework, Version 5.30, and its different modules. Impedance spectra were recorded at the open circuit potential (OCP) from 100 kHz down to 10 mHz, 10 points per frequency decade and signal amplitude of 5 mV rms. These spectra were obtained in different times of immersion (5 min, 24 h and 168 h) for the four buffer solutions. Polarization curves were done in a potential interval of -0.050 V (vs. OCP) until a potential where the anodic current was 500 μA. Mott-Schottky analysis were carried out at 1.63 kHz with signal amplitude of 10 mV rms. These spectra were recorded in 5 min and 168 h of immersion in the electrolytes. The Flat Band Potential, EFB, and Acceptor density, NA, were obtained considering that the major component of the passive film was Hg2Cl2 (Dielectric constant, ε = 9.4).
The UV assays were done on a 50 Conc Varian® UV-Visible Spectrophotometer using quartz cell to analyze the four electrolytes after 168 h of sample immersion comparing to a reference solution which had no contact with the sample. Ultra-pure water was the blank.
The X-Ray diffraction analyses were carried out on Shimadzu® X-Ray Diffractometer XRD-6000 with a Cu X-Ray source (λ=1.54060 Å), potency of 1.2 kW (40 kV and 30 mA) and scan rate of 2 degrees/min. The samples were prepared in an aluminum sample carrier and the diffractograms were immediately recorded between 20 and 120 degrees. The phases were identified with JCPDS (Joint Committee on Powder Diffraction Standards) database.
All the experiments were carried out at room temperature (25 ºC).
2.2 Electrolyte solutions
As discussed previously, four types of solutions were used: Phosphate Buffer Saline, PBS, Hank Solution, Articial Saliva and NaCl 0.9%. Preparation of 1 L of each of the solutions in which the electrodes were immersed was as follows:
- PBS (pH 6.80): 8.77 g NaCl, 3.58 g Na2HPO4, 1.36 g KH2PO4.
- Hank solution (pH 7.40): 8.00 g NaCl, 0.40 g KCl, 0.185 g CaCl2.2H2O, 0.06 g MgSO4.7H2O, 0.06 g Na2HPO4, 0.06 g KH2PO4, 0.10 g MgCl2.6H2O, 0.35 g NaHCO3, 0.50 g D-glicose.
- Articial Saliva (pH 6.80): 0.1256 g NaCl, 0.7632 g Na2SO4.10H2O, 0.9639 g KCl, 0.1780 g NH4Cl, 0.1892 g KSCN, 0.2278 g CaCl2.2H2O, 0.6545 g KH2PO4, 0.6308 g NaHCO3, 0.2000 g Urea, CO(NH2)2.
- NaCl 0.9%: 9 g NaCl.
All the solutions were prepared using ultra-pure water of resistivity > 18 MΩ cm and analytical grade reagents.
3. RESULTS
3.1 X-Ray Diffraction
The diffractogram of a freshly prepared sample of Tytin® Plus dental amalgam, is shown in Fig. 1a. The chemical microphases present on it are identified, and corroborate data from literature [2]. The amalgam Duxalloy® presented a similar chemical microstructure. Fig. 1 b shows the diffractogram of the amalgam Tytin® Plus after one week of immersion in PBS, where it can be observed the appearing of new diffraction peaks. This was also observed in presence of the other three types of electrolytes. These peaks can be attributed to the formation of a passive film (AgCl and/or HgCl2) on the surface of the amalgams, which is responsible for their semiconductive properties. These properties were measured by the parameters obtained from the electrochemical studies, discussed from now on in this work.
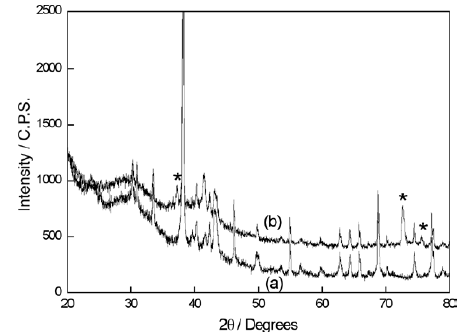
Fig. 1- Diffractograms of Tytin® Plus dental amalgam. (a) Freshly prepared; (b) after one week of immersion in PBS. The new peaks that appeared after the immersion are indicated with an asterisk (*).
3.2 Electrochemical Impedance Spectroscopy
Impedance spectra were analyzed by the software Gamry® Echem Analyst Version 5.30. The best fitting circuit consisted of a parallel combination RQ, and the solution resistance, RΩ, as shown in Fig. 2a. In other cases, the proposed model for the interface metal/passive film/solution is shown in Fig. 2b. Figure 2c e 2d show a physical representation of the two types of film formation that occurs over amalgam surface.
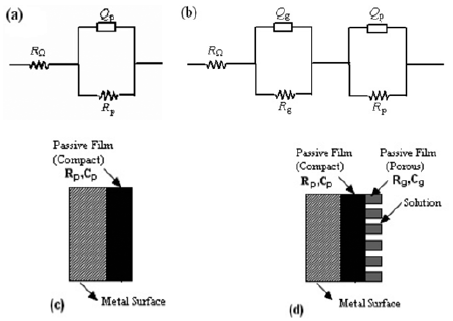
Fig. 2- Electric circuit used to simulate impedance data. (a) RW represents the solution resistance; Qp represents a constant phase element, CPE, for the capacitive behavior of the compact passive film; Rp represents the resistance of the passive film. (b) RW represents the solution resistance; Qp represents a constant phase element, CPE, for the capacitive behavior of the compact passive film; Rp represents the resistance of the compact passive film; Qg represents a constant phase element, CPE, for the capacitive behavior of the porous passive film; Rg represents the resistance of the porous passive film. (c) Physical representation of the passive film (one layer). (d) Physical representation of the passive film (bilayer).
The parameter RΩ is related to the resistance of the entire electrochemical environment, including solution resistance, wire connections resistance, etc. For Duxalloy® and Tytin® Plus, no significant variation of this parameter was observed with the immersion time except for artificial saliva which presented a value for RΩ four times bigger than the other electrolytes, therefore showing, comparatively, a lower conductivity.
The capacitance is the electric unit for a capacitor, Q, while the parameter α indicates the roughness factor being equal to 1 for a smooth and homogeneous surface. In general, the observed α values diverge from this ideal value, indicating a certain degree of roughness of the protector film.
In some cases, the electric circuit with one time constant (Fig. 2a) was necessary, suggesting the formation of a compact passive film. In the majority of cases, the electric circuit with two time constants (Fig. 2b) was needed, a fact that indicates the formation of bilayer passive film, composed of an external porous and one internal compact layer (Fig. 2c and 2d). An impedance spectrum and the fitting line are shown in Fig. 3a. Fig. 3b and 3c show the resistance of the compact passive film, Rp, obtained from the fitting, as a function of immersion time and electrolyte for the Duxalloy® and Tytin® Plus dental amalgams.
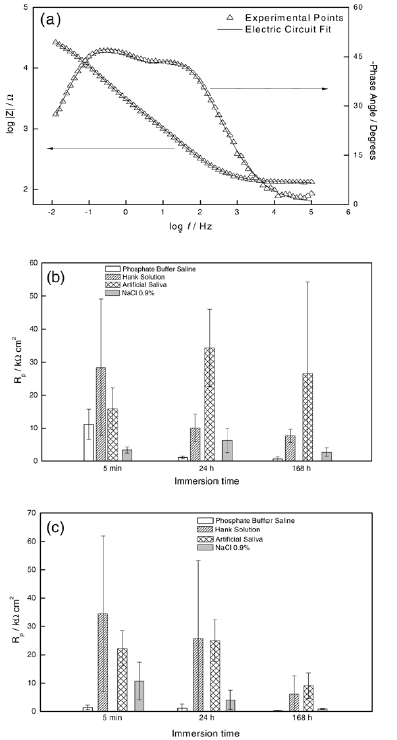
Fig. 3- (a) Representative Bode traces for the dental amalgams immersed in Artificial Saliva for 168 h. Rp in function of immersion time for the dental amalgams (b) Duxalloy® and (c) Tytin® Plus in the presence of different synthetic physiological fluids.
3.3 Polarisation curves
These curves were obtained to characterize the formation region of the passive film (if it appears) on dental amalgams, since the Mott-Schottky analysis are only possible if a passivation region is evident on the polarization curve. This region is observed on the curve where the current remains constant during a potential interval.
A visual inspection of the curves showed a passivation region on artificial saliva and PBS. Representative curves are shown as an example of the passivation region (Fig. 4a).
Corrosion potential and corrosion current, Ecor and icor, respectively, were obtained by the analysis of the polarization curves. These values show how much the alloy is susceptible to corrosion. A high Ecor and a low icor are essentials to inhibit corrosive processes. Fig. 4b-4e show comparative charts of -Ecor and icor in function of the immersion time and electrolyte for the Duxalloy® and Tytin® Plus dental amalgams.
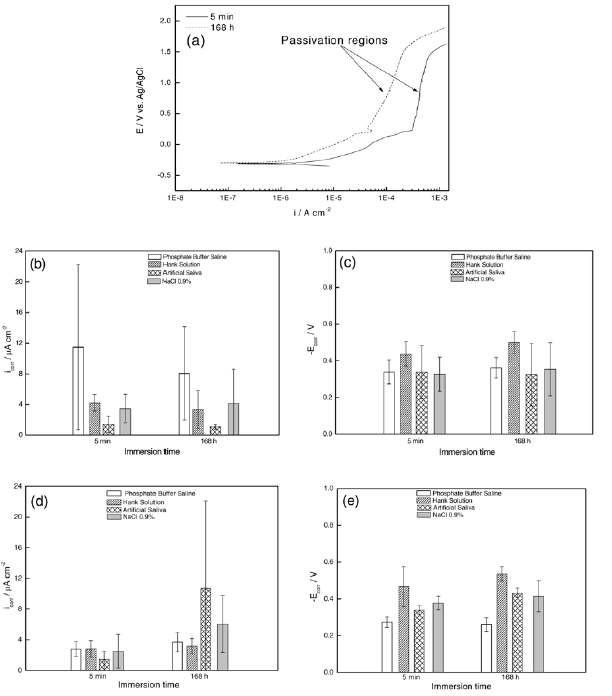
Fig. 4- (a) Polarisation curves with evident passivation regions of a Duxalloy® sample in PBS. Comparative charts of icor and –Ecor in function of the time of immersion and electrolytes for Duxalloy® (b, c) and Tytin® Plus (d, e) dental amalgams.
3.4 Mott-Schottky analyses
N-type semiconductors have, on its composition, atoms that have more electrons in their valence band than the matrix atoms (atoms present in majority). This extra electron is located at the top of space charge region. This top region is designated Fermi level and denotes the probability of electronic occupation of 0.50. P-type semiconductors have atoms that have fewer electrons in their valence band than the matrix atoms. Therefore, the activation of electrons is more effectively to a level of energy near the valence band and inside the space charge region. This is the Fermi level for a P-type semiconductor. So, N-type and P-type semiconductors provide negative and positive carriers, respectively [7].
The Mott-Schottky analyses are complementary to EIS and polarization curves where, at high frequencies (kHz), it is possible to set a predetermined potential gap and measure the passive film capacitance which has a semiconductive behavior. The experimental result is a curve of the reciprocal of the squared capacitance of the space charge (1/CSC2) versus the potential (see Fig. 5a). From this curve, it is possible to determine the Flat Band potential, EFB, extrapolating to the abscissa (C = 0), and the donor and acceptor densities, ND and NA respectively. A greater density of donor or acceptors implicates in major charge transfers inside this film increasing the probability of reactions occurring at the surface of the metal. This results in an increase of the film thickness [8]. For both brands of dental amalgams, a decrease of the EFB and NA was observed with the increase of immersion time in PBS and artificial saliva (Fig. 5b-5e).
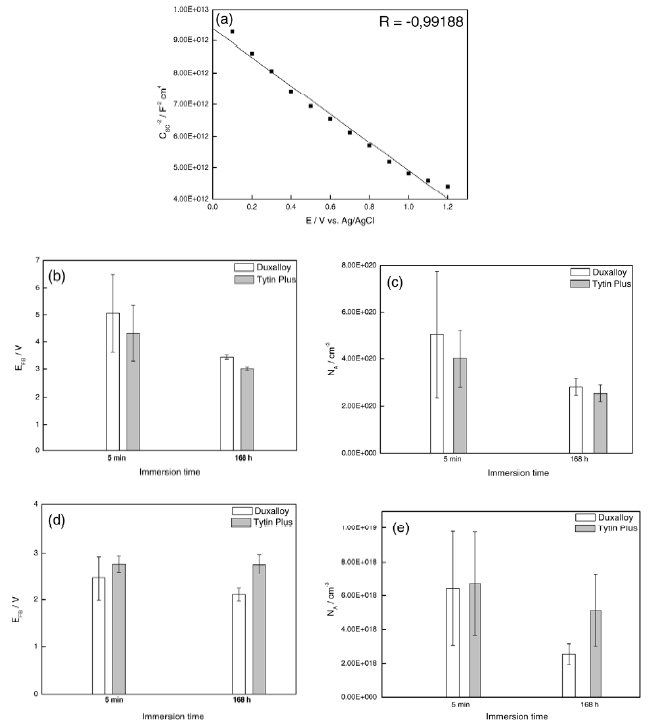
Fig. 5- (a) Mott-Schottky plot for Duxalloy® sample in artificial saliva after 5 minutes of immersion. Flat Band potential, EFB, and density of charge acceptors, NA, for Duxalloy® and Tytin® Plus samples immersed in PBS (b, c) and in artificial saliva (d,e).
3.5 UV spectrophotometry
Figure 6 shows the absorption spectra of NaCl 0.9% after immersion of dental amalgams for 168 h. Relating to the reference sample (solution which had no contact with the amalgams), a discreet absorption band is observed at ~276 nm. The same behavior was observed for the other electrolytes (PBS, Hank Solution and Artificial Saliva).
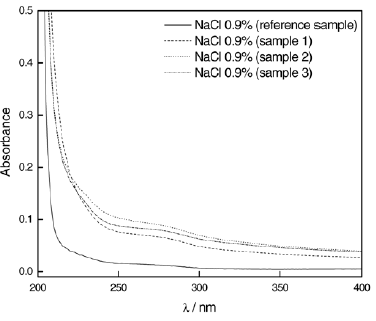
Figure 6 - UV spectra after 168 h of immersion of Duxalloy® samples in NaCl 0.9%.
4. DISCUSSION
From the analysis of Figure 1a and 1b, no significant difference was found among the two amalgams. The presence of the γ2 phase was not observed, confirming the expectations of high copper alloys. This result is according to Brett et al. [9]. Figure 4 shows the behavior of Rp (compact film resistance) for the two brands of dental amalgams in function of the immersion time and the type of electrolyte. The minor corrosion resistance of the amalgams in NaCl 0.9% and phosphate buffer solution might be due to the high concentration of chloride anions, making them aggressive electrolytes. Another noticeable fact is that Duxalloy® exhibited a major resistance to corrosion when immersed in artificial saliva. Regardless of having the lower pH value, which would theoretically make it the most aggressive electrolyte, the most protective film was formed in this medium since its composition might have Ag2SO4, AgCl and Hg2Cl2, compounds that are insoluble salts [1, 4]. Tytin® Plus showed the highest corrosion resistance in Hank solution at low immersion times. The high chloride concentration is combined with a high pH value which, in this case, lessens the diffusion of silver, copper and tin ions into the medium [10]. High pH values also decrease the formation of tin oxides and chlorides which diffuse to the bulk of solution generating pores and low corrosion resistance [4]. Therefore, the passive film formed in Hank solution has the best capability to avoid corrosion.
Being the dental amalgam a biomaterial used for a long period, the immersion time of 168 h is considered the most representative of the expected behavior in oral cavity. For Duxalloy®, Rp did not change significantly after 24 h of immersion and the major changes were observed from 5 min until 24 h; for Tytin® Plus, Rp decreased significantly after 24 h of immersion, fact that occurred between 5 min and 24 h of immersion. In the period of 24 h until 168 h of immersion, a global decrease of resistance took place and can be explained by attacks of the electrolytes on the passive film resulting in its rupture. A major decrease was observed for Tytin® Plus when compared to Duxalloy®.
The corrosion of dental alloys in the oral cavity has its origin on diverse species such as chloride and hydrogen ions, sulfides, oxygen and microorganisms [6]. All the electrolytes present some of these species in their composition.
From Fig. 4b-4e, the following considerations were made:
- Duxalloy® samples exhibited the following decreasing order of corrosion resistance:
- 5 min: Artificial Saliva > NaCl 0.9% > Hank solution > PBS.
- 168 h: Artificial Saliva > Hank solution > NaCl 0.9% > PBS.
- Tytin® Plus samples exhibited the following decreasing order of corrosion resistance:
- 5 min: Artificial Saliva > PBS ~ NaCl 0.9% ~ Hank solution.
- 168 h: Hank solution > PBS > NaCl 0.9% > Artificial Saliva.
For Duxalloy®, the immersion time did not show great influence on icor values. Comparatively, with 168 h of immersion, this corrosion resistance decreasing order corroborates the impedance data discussed earlier. As seen above, PBS has the highest icor value, fact which can be explained by the high content of chloride anions provided by NaCl, low pH value or the possibility of formation of a film with weak passive properties [11]. Contrarily to the observed behavior of Duxalloy® samples, Tytin® Plus had the icor values increasing with the increase of immersion time, especially in the presence of artificial saliva. This high icor value is related to the presence of thiocyanate ions, which enhances the corrosion processes of chloride anions [11]. Notice that, in this case, a lower corrosion current was observed in PBS when compared to Duxalloy®, indicating that the film was much capable of decreasing corrosion reactions. Although the electrolyte order of corrosion resistance for 168 h does not match completely with impedance data, the polarization curves show that Tytin® Plus sample are more susceptible to corrosion when compared to Duxalloy® samples.
Referring to the polarization curves (see Fig. 4a), evident formation of passive films was noted on artificial saliva and PBS. This region had a reasonable size (~ 1 V), making these electrolytes optimums for the Mott-Schottky studies.
The experimental curves showed that the passive films formed over the amalgams exhibited a P-type semiconductive property which is confirmed by the negative slopes of the Mott-Schottky curves (Fig. 5a) [7]. A decrease of EFB and NA is also noticed with the immersion times for both brands of dental amalgams and for both electrolytes (Fig. 5b-5e). As these two terms are intimately related with the thickness of the formed film, the semiconductive behavior decreases and, consequently, its passive capability. This data corroborates with EIS data which shows a decrease of the passive film resistance with the increase of immersion time in artificial saliva and PBS.
UV spectrophotometry showed a band at ~276 nm that may be related to the formation of chloride-complexes with the constituent metals of the amalgam, being the Cl- a localized corrosion causing agent of metallic biomaterials. Since chloride anions are present in the chemical composition of the solutions, this fact indicates a possibility of a metal dissolution event, justified by the appearance of the absorption band (see Fig. 6).
5. CONCLUSION
All data lead to the same result: the passive film resistance is high at initial immersion times and decreases after 168 h. Although this fact is systematic, the passive film continued to show its semiconductive and resistive properties even after a period of 168 h of immersion, in other words, it presented protective properties against corrosion and because of this, the biomaterial does not produce potential risks to human health.
Future studies should take into account the influence other synthetic physiological fluids, the effect of temperature and pH.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Brazil (Processes CDS 12/03 and cex 1736/06).
REFERENCES
1. C. M. A. Brett, I. Ioanitescu, F. Trandafir, Cor. Sci. 46 (2004) 2803. [ Links ]
2. M. Marek in J. Scully, Treatise on material science and technology, ed. Academic Press, London, 1983, England.
3.M. Marek, Adv. Dent. Res. 6 (1992) 100.
4. H. A. Acciari, A. C. Guastaldi, C. M. A. Brett, Cor. Sci. 47 (2005) 635.
5. C. M. A. Brett, F. Trandafir, J. Electroanal. Chem. 572 (2004) 347.
6. V. A. Alves, L. A. da Silva, L. M. da Silva, A. Rossi, L. F. F. Santos, D. T. Cestarolli, J. Appl. Electrochem. 37 (2007) 961.
7. A. W. Bott, Curr. Sep. 17 (1998) 87.
8. H. X. Guo, B. T. Lu, J. L. Luo, Electrochim. Acta 52 (2006) 1108.
9. C. M. A. Brett, H. A. Acciari, A. C. Guastaldi, Key Eng. Mater. 230-232 (2002) 463.
10. G. Bayromoğlu, T. Alemdaroğlu, S. Kedici, Aksüt, J Oral Reh 27: (2000) 563.
11. M. F. L. Mele, G. Duffó, J. Appl. Electrochem. 32 (2002) 157.













