Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.3 Lisboa set. 2018
CASE REPORT
Arterio-arterial graft – an option for hemodialysis patients with exhaustion of venous patrimony
Ana Castro1,2, Paulo Almeida3, Filipa Silva1, Duarte Rego3, Joana Tavares1, Josefina Santos1, Fernanda Silva1, José Alexandre Queirós1, António Cabrita1, Rui Almeida3
1 Serviço de Nefrologia, Centro Hospitalar do Porto, Porto, Portugal
2 Unit for Multidisciplinary Research in Biomedicine, Porto, Portugal
3 Serviço de Cirurgia Vascular, Centro Hospitalar do Porto, Porto, Portugal
ABSTRACT
Introduction: Vascular access (VA) for hemodialysis (HD) is the lifeline for End Stage Renal Disease (ESRD) patients. Long-term HD patients often have exhaustion of their venous patrimony for an autologous VA construction and, sometimes, even for a central venous catheter (CVC) placement.
Case report: We describe the case of a 43-year-old woman with ESRD due to lupus nephritis, on maintenance HD since 2009. She also had secondary antiphospholipid syndrome and was chronically anticoagulated. Nevertheless, the patient had multiorgan thrombotic events (without sequelae) and several episodes of irreversible thrombosis of arteriovenous fistulas. Her HD course was also marked by multiple severe CVC infections, at different locations; a hemoperitoneum during cholecystectomy, and an immediate thrombosis of the renal artery of a kidney transplant. She was admitted to our hospital after an irreversible dysfunction of a right jugular CVC, with documentation of thrombosis of the superior and inferior vena cava. Exhaustion of the venous patrimony for HD was assumed and it was decided to make an arterio-arterial graft (AAG) of early cannulation. The first cannulation of the AAG was performed two days after surgical intervention, with no complications. The patient performed a twelve hour per week HD treatment with good efficiency.
Conclusion: AAG is an alternative for HD patients who have exhausted all their venous patrimony and it can be considered prior to the placement of a CVC as their sole remaining vascular access.
Key Words: arterio-arterial graft; early cannulation graft; autologous vascular access; hemodialysis
INTRODUCTION
Worldwide, approximately 10% of the population has chronic kidney disease (CKD) and about 1.5 million require hemodialysis (HD)1.
The prevalence of ESRD patients requiring renal replacement therapy (RRT) is continuously increasing and is expected to reach 5.4 million worldwide by 20301. Most patients are treated with HD, which in most countries exceeds 60% of the prevalent ESRD population2.
Vascular access (VA) for hemodialysis is the lifeline for ESRD patients and arteriovenous fistula (AVF) is the preferred VA since it is associated with less complications. However, long-term HD patients often require multiple VA interventions. In Europe over 25% of all hospitalizations observed in ESRD patients are related with the construction and maintenance of a patent HD VA3.
When all the venous capital of an HD patient is exhausted, the use of an arterial access for HD may be a viable option and, in fact, the use of an artery as a permanent VA is not a new procedure.
In this article we describe the clinical case of a patient with ESRD in HD treatment with venous capital exhaustion, including for central venous catheter (CVC) placement, for whom our team first arterial brachio-brachial graft of early cannulation was constructed.
CASE REPORT
We describe the case of a 43-year-old woman with ESRD due to lupus nephritis (LN) on maintenance HD since 2009. She was diagnosed with systemic lupus erythematous (SLE) in 1993 when she was admitted at our hospital with a severe presentation of the disease, with cutaneous, haematological, hepatic, renal and pulmonary involvement. Immunosuppressive therapy with cyclophosphamide and corticosteroids was started with favorable clinical evolution. Posteriorly, she was also diagnosed with secondary antiphospholipid syndrome (APS) and despite the treatment with hypocoagulation she had several thrombotic events (cerebellar infarcts, pulmonary thromboembolism) without neurological deficits or other sequelae.
She evolved with progressive decline of renal function and severe arterial hypertension requiring the initiation of RRT by a tunneled CVC in the right internal jugular vein (RIJV) in February of 2008. Despite the close follow-up in our Vascular Access Consultation (VAC) and the multidisciplinary approach by Nephrology and Vascular Surgery, a complex path of vascular accesses followed (Fig.1).). In 2008 a left brachiocephalic AVF was constructed, via which the patient began HD. However, she had a thrombosis without recovery in 2009 and this access was abandoned. This was followed by a complex course of multiple CVCs, with different locations and recurrent infectious complications. A new autologous VA (right brachiobasilic AVF) was constructed, but it also irreversibly thrombosed, and therefore, had to be abandoned. The total absence of venous patrimony compatible with the construction of an autologous VA in the upper limbs was confirmed after a new VAC evaluation.
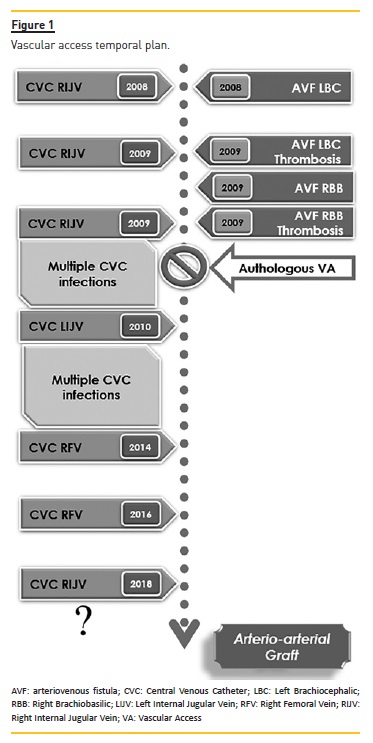
In 2010 the patient had an acute lithiasic cholecystitis and underwent a laparoscopic cholecystectomy. Nevertheless, it was complicated with the hemoperitoneum requiring posterior laparotomy.
The patient was submitted to a deceased donor kidney transplant in 2011 with immediate thrombosis of the renal artery, and the kidney graft had to be removed.
In February of 2018 the patient had an infectious complication of the right femoral CVC (abscess of the tunnel that fistulized to the skin), and was referred by the HD center to our hospital to remove it and place a new one. Given the previous trajectory, it was decided to try to place, once again, an echo-guided and radioscopically controled with contrast infusion CVC.
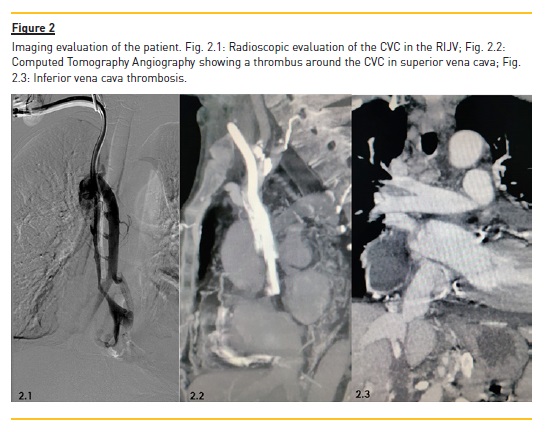
A 19cm long-term catheter Cannon® II from Arrow with 15Fr was placed in the RIJV. Although the CVC progression was very difficult to perform, it was considered, at that point, the last feasible VA for the patient (Fig.2).
A first HD treatment was performed at the hospital, with no complications. After three HD treatments the CVC presented with irreversible dysfunction, probably due to its location.
The patient was hospitalized for study and therapeutic orientation. Conservative therapy for CKD was immediately started and patient clinical and analytical surveillance was performed daily. The need for HD access was urgent.
A computed tomography angiography (CTA) of the thorax and abdomen was performed to characterize the entire vasculature, revealing that the CVC was introduced into the right internal jugular vein , the distal tip of which was in the most distal region of the superior vena cava (Fig.2). The entire upper vena cava had a thrombus around the catheter, without identification of the vessel wall or lumen. Upstream, the brachiocephalic venous trunks were permeable. An important collateral circulation in the thoracic wall associated with the chronic superior vena cava thrombosis was seen.
Surprisingly, it was also observed that the inferior vena cava was also completely obstructed, only permeable above the confluence of the suprahepatic veins.
Taking all these findings into account, we considered that the patient had exhaustion of the venous patrimony compatible with a vascular access for HD.
In this way, the clinical case was discussed with the Peritoneal Dialysis (PD) Unit to consider the possibility of performing PD. All the hospitalizations and surgical interventions related to the previous cholecystectomy, renal transplantation and all the intra-abdominal haemorrhagic/thrombotic complications that followed were carefully reviewed. It was concluded that after all episodes of intra-abdominal bleeding and laparotomies (with risk of associated adhesions), the technique had only a small chance of sucess.
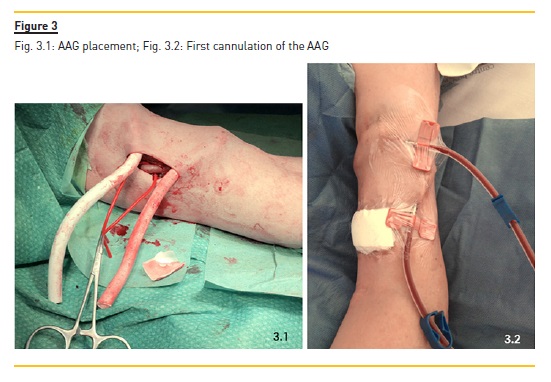
After a multidisciplinary – Nephrology and Vascular Surgery – re-discussion of the patient, and considering the preservation of the thoracic arterial patrimony, the construction of an arterio-arterial graft (AAG) of early cannulation was proposed. A brachio-brachial (with end-to-end anastomosis) loop, using a 6 mm graft, was implanted in the left upper limb (Fig.3.1). At the end of the surgery the AAG pulse was present and the brachial pulse was also patent. No complications were reported.
Some particularities in the management of this type of graft were reviewed by the hemodialysis team: the puncture is in ladder, to prevent the creation of false aneurysms since there is very high venous pressure; it is not possible to perform hemodiafiltration since the inflow is made into the arterial circuit and would induce ischemia; the administration of drugs in the graft is contraindicated; more prolonged hemostasis time; Qb is limited by venous pressure, so in heavier patients an adequate dialysis dose will only be possible with 4 dialysis sessions or 3 long sessions.
The first cannulation was performed by an experienced HD nurse two days after the procedure (Fig.3.2) using two 17G needles. The patient underwent a fourhour HD treatment without intercurrences. At the end a mean hemostasis time of approximately 30 minutes was observed. The patient was discharged to the hospital 1 week after surgery, during which dialysis therapy, namely ultrafiltration, was optimized. She maintained oral anticoagulation. Given the particular characteristics of the VA, the patient continued treatment in the HD unit of our hospital. She was treated with 12h of dialysis per week with mean Kt/V of 1.2, blood flow of 250-300 ml/min, an arterial pressure ranging from -70 to -50 mmHg and venous pressure ranging from 200 to 250 mmHg. Her height was 150 cm and dry weight was 44Kg, which permitted three treatments per week.
Two months after the procedure the patient was admitted with septic shock secondary to left lower limb cellulitis, and died in this context .
DISCUSSION
With the improved life expectancy of HD patients, we are confronted with patients who have exhausted their possible venous sites for AVF creation and arteriovenous graft (AVG) or even CVC placement.
In this article, the authors report the case of a young woman with APS, with multiple failure of autologous VA in the upper limbs due to thrombosis. In addition to the depletion of superficial and deep venous capital in the upper limbs, the construction of any access at this location was compromised due to occlusion of the superior and inferior vena cava. Although attempting to construct an AVF at the lower limb level was feasible, the likelihood of thrombosis was extremely high.
Whilst in 2010 the possibility of constructing this type of AV existed, the practices and access policy at that time were quite sparse and different from those currently implemented. Even for urgent situations, the response time was very long; at that time the only solution found was a CVC placement.
The sites available for catheter placement are finite and should be used sparingly. As we saw with this case, complications associated with long-dwelling catheters are frequent and inevitably lead to venous patrimony losses through time. In the femoral site we used the 50cm long-term catheter Cannon II from Arrow with 15Fr.
However, since the patient had a severe infectious complication in her last right femoral CVC and a complete obstruction of the inferior vena cava was observed, the possibility of a successful introduction of a new femoral CVC was excluded.
Although stenosis and thrombosis of central veins can be treated by angioplasty or surgically, these procedures require a suitable jugular vein or a patent contralateral venous outflow. Previous studies have demonstrated that treatment of central venous occlusions by angioplasty shows an excellent initial result, but 1-year primary patency rates are ≤ 50%; restenosis rates are high, and long-term results are uncertain4-10. Given the severe AFS and all the previous thrombosis under anticoagulation, neither angiographic or surgical treatment of the central venous occlusions were performed. In order to preserve the venous entry site, the catheter exchange could be made through a guidewire, using, for example, a CVC with a reduced lumen CVC. However, in this case, given all the serious infectious complications, this possibility was not considered. We tried a recanalization, through a CVC placement, but it was not successful. It was possible to place the CVC in the right atrium by collaterality but the Qb was not adequate for HD.
In patients who have exhausted all possible conventional means for an autologous VA, an AAG can be the only other option for hemodialysis. In fact, the use of an artery as permanent vascular access for HD is not a new procedure.
The AAG was first described by Butt and Kountz in 197611. They reported good results with a femoropopliteal graft employed in seven patients in whom a conventional VA for HD was not possible. Since that time, a total of eight main retrospective studies were published between 1976 and 2017, reporting data of 151 patients12. Primary patency rate ranged from 67%-94.5% at six months to 54%-61% at 36 months; secondary patency rates from 83%-93% at six months to 72%-87% at 36 months12.
The main indication for the AAG is inappropriate venous capital, such as absence of superficial venous capital, absence of deep veins surgically accessible and obstructive central venous disease (intractable or recurrent).
Other indications for AAG that can be considered are the risk of developing cardiac heart failure or high risk of critical ischaemia of the extremity with other VA.
AAG can be placed in three main regions: axillary, brachial and femoral. The axillary AAG involves the need for general anesthesia and their cannulation is worse, particularly in women. On the other hand, brachial AAG allows for easier cannulation and placement can be made under general or local anesthesia. However, it is only possible to use arteries with diameter greater than 4 mm. Femoral AAG allows better flow, but has a higher risk of infection.
Although all studies reported satisfactory haemodialysis, only Bunger at al13 and Zanow et al14 described the actual dialysis data. In the first study the blood flow ranged between 250 and 300 ml/min and in the second 272 mL/min for axillary loops and 416 mL/min for femoral loops. In Zanow et al study there was a four-hour treatment efficiency. Given the fact that our patient had about 50 Kg, a four-hour treatment three times per week had enough efficacy. However, two patients required five hours of treatment.
The hemostastatic compression ranged between 10 and 15 minutes14,15 and it was greater than 15 minutes in two studies16,17.
The post-operative management is different according to the published studies (Table 1) and there is no consensus about it12. In the published studies, the authors approached the anti-coagulation, the first needle puncture and the surveillance differently. In our patient we performed the first needle puncture 2 days after the AAG construction. She was already with anticoagulation.
Unfortunately, in our case report the patients short follow- up period was the limiting factor in VA monitoring.
One of the main complications associated with the construction of AAG, given the arterial pressures, is the risk of development of false aneurysms. In this case, grafts with triple layer may confer an advantage. Thromboembolism or pharmacological embolism are also possible complications in AAG, so drug administration is contraindicated in this form of vascular access. However, the most feared complication identified is distal ischemia associated with graft occlusion. Interestingly, in the studies performed the manifestations of ischemia were not concordant13,17.
Nevertheless, Zanow et al noted that thrombosis of the femoral AAG required immediate thrombectomy14. Thus, it may be reasonable to consider that in femoral AAG, thrombosis is associated with a more severe ischemia, so revascularization must be immediate. In the AAG of the upper limb, ischemia is more tolerable (probably due to collateral circulation), so revascularization may be delayed.
Our patient died of septic shock secondary to left lower limb cellulitis. Although the possibility of septic embolization could be considered, the AAG had no local infectious signs and there was an entrance door in the outer side of the left leg, making this the most probable starting point for the infection.
CONCLUSION
Although there is a lack of evidence on the use of AAG, the data obtained from the studies performed is encouraging. This is an alternative VA for HD patients who have exhausted all their definite access sites which can be considered prior to the placement of a CVC as their sole remaining VA. However, there is a need for a multicenter study with a larger population of patients.
References
1. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385(9981):1975-82. [ Links ]
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017; 69(3S1):A7-A8. [ Links ]
3. Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, et al. Mortality and hospitalization in hemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2004; 19:108-20. [ Links ]
4. Dammers R, de Haan MW, Planken NR, van der Sande FM, Tordoir JH. Central vein obstruction in hemodialysis patients: results of radiological and surgical intervention. Eur J Vasc Endovasc Surg 2003; 26: 317-21. [ Links ]
5. Lumsden AB, MacDonald MJ, Isiklar H, Martin LG, Kikeri D, Harker LA, et al. Central venous stenosis in the hemodialysis patient: incidence and efficacy of endovascular treatment. Cardiovasc Surg 1997; 5:504-9. [ Links ]
6. Kalra M, Gloviczki P, Andrews JC, Cherry KJ Jr, Bower TC, Panneton JM, et al. Open surgical and endovascular treatment of superior vena cava syndrome caused by nonmalignant disease. J Vasc Surg 2003; 38:215-30. [ Links ]
7. Kovalik EC, Newman GF, Suhocki P, Knelson M, Schwab SJ: Correction of central venous stenoses: use of angioplasty and vascular wall stents. Kidney Int 1994; 45:1177-81. [ Links ]
8. Aytekin C, Boyvat F, Yagmurdur MC, Moray G, Haberal M. Endovascular stent placement in the treatment of upper extremity central venous obstruction in hemodialysis patients. Eur J Radiol 2004; 49:81-5. [ Links ]
9. Verstandig AG, Bloom AI, Sasson T, Haviv YS, Rubinger D. Shortening and migration of Wallstents after stenting of central venous stenosis in hemodialysis patients. Cardiovasc Intervent Radiol 2003; 26:58-64. [ Links ]
10. Smayra T, Otal P, Chabbert V, Chemla P, Romero M, Joffre F, et al. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol 2001; 24:388-94. [ Links ]
11. Butt KM, Kountz SL. A new vascular access for hemodialysis: the arterial jump graft. Surg 1976; 79(4):476-9. [ Links ]
12. Grima MJ, Vriens B, Holt PJ, Chemla E. An arterioarterial prosthetic graft as an alternative option for haemodialysis access: a systematic review. J Vasc Access 2018 Jan; 19(1):45-51. [ Links ]
13. Khafagy T, Regal S, ElKassaby M, Saad E. Early results of brachial arterio-arterial prosthetic loop (aapl) for hemodialysis. Eur J Vasc Endovasc Surg 2016; 51(6):867-871. [ Links ]
14. Lei W, Ji J, Wang J, Jin L, Zou H. Arterioarterial prosthetic loop as an alternative approach for hemodialysis access. Medicine (Baltimore) 2015; 94(41):e1645. [ Links ]
15. Bünger CM, Kröger J, Kock L, Henning A, Klar E, Schareck W. Axillary-axillary interarterial chest loop conduit as an alternative for chronic hemodialysis access. J Vasc Surg 2005; 42(2):290-95. [ Links ]
16. Moncef G. Arterio-arterial graft interposition and superficial femoral vein transposition: an unusual vascular access. Saudi J Kidney Dis Transplant 2005; 16(2):171-75. [ Links ]
17. Zanow J, Kruger U, Petzold M, Petzold K, Miller H, Scholz H. Arterioarterial prosthetic loop: a new approach for hemodialysis access. J Vasc Surg. 2005;41(6):1007-12. [ Links ]
18. Talaiezadeh AH, Haghighi KE. Arterio-arterial prosthetic duct (AAD) as a vascular access in hemodialysis. J Vasc Access. 2004; 5(3):113-5. [ Links ]
19. Giacchino JL, Geis WP, Buckingham JM, Vertuno LL, Bansal VK. Vascular access: long-term results, new techniques. Arch Surg. 1979; 114(4):403-9. [ Links ]
20. Butt KM, Kountz SL. A new vascular access for hemodialysis: the arterial jump graft. Surgery. 1976; 79(4):476-9. [ Links ]
Ana Castro, MD
Serviçode Nefrologia, Centro Hospitalar do Porto
Largo Prof. Abel Salazar,4099-001 Porto, Portugal
E-mail: ana.coutinho.castro@gmail.com
Received for publication: Jul 6, 2018
Accepted in revised form: Sep 20, 2018














