INTRODUCTION
The burden of chronic kidney disease (CKD) is increasing, with prevalence ranging from 5 to 13% among countries.1 Worldwide, there are more than 750 million people with CKD2 and, between 2016 and 2040, the CKD prevalence is expected to jump to 5th place in the ranking of the main causes of early death.3 In 2017, Chronic Kidney
Disease (CKD) was responsible for the 12th highest number of fatalities worldwide, accounting for 1.2 million deaths4. In Brazil, the prevalence of CKD is estimated to range from 3 to 6 million cases5. Furthermore, in 2016, CKD held the rank of 9th among the leading causes of mortality in the country6.
In addition to the impact on health, CKD has economic and social repercussions.7 Between 2013 and 2015, about 2% of hospitalizations and 3% of the expenditures of the Unified Health System (UHS) were due to CKD with a growth of approximately 12% in complementary tests and 11% in expenditures on disease identification and management.8 Dialysis establishments in the country increased by about 38% between 2002 and 2017, in line with the 159.4% increase in the number of patients.9 Furthermore, socioeconomic disparities contribute to the high rate of CKD in less favored populations.2,10
Diabetic nephropathy and hypertensive nephrosclerosis are the main causes of CKD in developed and developing countries.11,12 Arterial hypertension (AH) is found in up to 9 in every 10 CKD patients13 and diabetic nephropathy in up to half of the higher stages only in the USA.14 The increase in serum uric acid (UA) is considered a risk factor for the development of AH, diabetes mellitus (DM), dyslipidemia, obesity and cardiovascular events15 and has a high prevalence in CKD.16,17
The risk of CKD accelerates with UA levels above 6-7 mg/dL in women and 7-8 mg/dL in men.18 However, there is still no verdict on whether UA is a predictor of the incidence of CKD or a biomarker of its progression.19 Even so, it is relevant to monitor and control the level of UA in order to delay the functional decline of the kidneys,20 especially in patients at high risk,21 because high levels of UA may represent an opportunity for preventive intervention in both incidence22 and progression of CKD.23
In this context, the aim of this study was to identify the prevalence of CKD and its association with serum UA levels in hypertensive and/or diabetic patients registered in a Basic Family Health Unit (BFHU).
MATERIAL AND METHODS
Study design and population
This is a cross-sectional study conducted with hypertensive and/or diabetic users registered in a BFHU, between 2019 and 2020.
The sample was defined considering the survey of the reference population with AH and DM (n = 647), prevalence of 15.4% of the phenomenon studied,24 margin of sampling error of 5% and confidence level of 95%. The sample calculation was performed through the GPower 3.1 program and resulted in a minimum sample size of 154 individuals, adults (age ≥ 18 years), hypertensive and/or diabetic who were selected by convenience during outpatient consultations. Children, pregnant women, patients with a history of alcohol or drug abuse, users with absent or incomplete data or no diagnostic confirmation were excluded. At the end, 182 individuals had available data and participated in the present study.
Variables and data collection
The review of the medical records allowed obtaining clinical parameters (presence of AH, DM, cardiovascular diseases (CVD), dyslipidemia, diuretic, angiotensin receptor blocker (ARB), angiotensin-converter enzyme inhibitor (ACEI), beta-blocker, calcium channel blocker (CCB) and lipid-lowering), behavioral variables (smoking, alcohol consumption) and laboratory data (fasting glycemia, total cholesterol, high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), triglycerides, serum creatinine, and serum UA).
Moreover, the global risk score (GRS) was calculated, which is used to estimate the probability of developing coronary, cerebrovascular, peripheral vasculopathy or heart failure in the next 10 years. Stratified at low risk (if GRS < 5%), intermediate risk (if GRS between 5% and 20% for men, between 5% and 10% for women, diabetic patients without risk stratifications or subclinical atherosclerosis disease), high risk (if GRS > 20% in men, GRS > 10% in women, presence of subclinical atherosclerosis or risk aggravating diseases) and very high risk (if important atherosclerotic disease with or without clinical repercussion).25
AU was the main exposure and its values were measured in milligrams per deciliter (mg/dL). CKD was the main outcome of this study and was defined as a kidney alteration for more than 3 months manifested as glomerular filtration rate (GFR) below 60 mL/min/1.73 m2. GFR was estimated using the CKD-EPI formula and classified, in mL/min/1.73m2, in stages G1 (GFR ≥ 90), G2 (GFR 60-89), G3a (GFR 45-59), G3b (GFR 30-44), G4 (GFR 15-29) and G5 (GFR < 15).26
Statistical analysis
The qualitative variables were described as absolute and relative frequencies and quantitative variables through measures of central tendency (mean) and variability (standard deviation). The association between CKD, UA and explanatory variables was based on the univariate and multivariate logistic regression model. Thus, in addition to the crude model, in model 1, an adjustment was made for age and gender. In model 2, additional adjustment was proposed for the main risk factors of CKD, i.e., AH, DM, CVD, dyslipidemia and lifestyle (smoking and alcohol consumption). In model 3, adjustment was added for laboratory variables (fasting glycemia, total cholesterol, HDL-c, LDL-c, triglycerides). Finally, model 4 was adjusted for the use of medications (thiazide diuretic, ABR, ACEI, beta-blocker, CCB and lipid-lowering).
RESULTS
Sample characteristics
The sample of 182 individuals was composed mostly of elderly people (63.82 ± 11.87 years), female (61%), non-black (76.4%), hypertensive (94.5%), non-diabetic (59.9%), with dyslipidemia (67%), nonsmokers (89.6%) or alcohol-consumers (94.5%) and with high cardiovascular risk (70.3%). The mean GFR was 79.88 ± 20.18 mL/min/1.73 m² and UA was 4.88 ± 1.83 mg/dL.
In general, the prevalence of CKD was 17% (95% CI = 12.1 - 23.3), affecting elderly people (71 ± 10 years), female (± 61.2%). In the stratification of global cardiovascular risk, the majority (26 patients) was classified as high risk and over 20% of patients with very high cardiovascular risk had CKD. In the CKD group, the mean plasma UA level was 5.7 ± 1.7 mg/dL (on average, 1 mg/dL higher compared to patients without CKD), serum creatinine of 1.28 ± 0.27 mg/dL and GFR of 49.91 ± 8.10 mL/min/1.73 m². There was a statistically significant difference for age, loop diuretic, creatinine, GFR and UA between the group with and without CKD (Table 1).
Table 2 shows that, in the univariate analysis, serum UA was positively associated with CKD (OR = 1.34 [95% CI = 1.09-1.64], p = 0.006).
After adjustment for the confounding variables, the association was maintained (ORa = 1.65 [95% CI = 1.11-2.45], p = 0.013). The increase of 1 mg/dL in UA increases the chance of the individual to have CKD by 1.65 times.
DISCUSSION
The prevalence of CKD in the studied population was 17% (95% CI = 12.1 - 23.3). The estimate of the prevalence of CKD in Brazil is imprecise, varying in different studies according to the population and methods of diagnosis of the disease.5 One study with the general population, which considered GFR <60 mL/min/1.73 m2 and/or albumin-creatinine ratio ≥ 30 mg/g, in a single measurement, found a value of 8.9,27 close to the overall prevalence of 9.1%.4 In the elderly (≥ 60 years), the prevalence of CKD based on data from the National Health Survey (NHS) was 21.4%, which corresponded to approximately three times the prevalence in adults.28
Table 1 Demographic, clinical behavioral and biochemical variables of the participants.
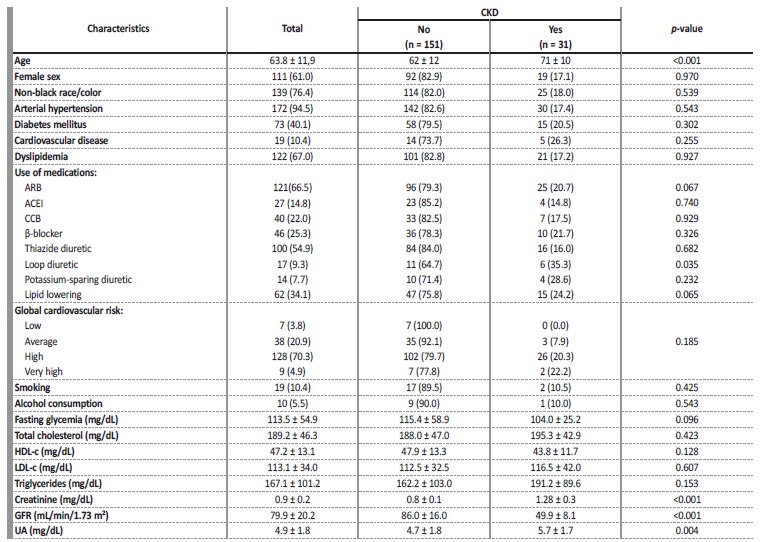
Values expressed as a percentage N (%) or mean ± standard deviation. N - absolute number; n - total cases; CKD - chronic kidney disease; ARB - angiotensin receptor blocker; ACEI - angiotensin-converting enzyme inhibitor; CCB - calcium channel blocker; HDL-c - high density lipoprotein cholesterol; LDL-c - low density lipoprotein cholesterol; GFR - glomerular filtration rate; UA - uric acid.
Table 2 Uni and multivariate analyses between serum UA and CKD.
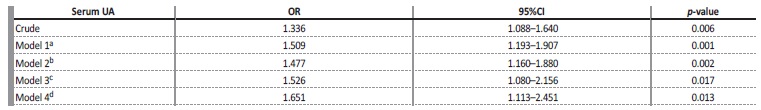
CKD - chronic kidney disease; UA - uric acid; OR - odds ratio; 95%CI - confidence interval
a Model 1 - adjusted for age and sex
b Model 2 - adjusted for model 1 + systemic arterial hypertension, diabetes mellitus, cardiovascular diseases, dyslipidemia, smoking, alcohol-consumption.
c Model 3 - adjusted for model 2 + fasting glycemia, total cholesterol, HDL-c, LDL-c, triglycerides.
d Model 4 - adjusted for model 3 + thiazide diuretic, angiotensin-receiver blocker, angiotensin-converting enzyme inhibitor, calcium channel blocker, beta-blocker, lipid lowering.
The prevalence found in our study was similar to the prevalence of 17.3% found in another cross-sectional study in 243 patients with AH and DM from a medium-sized city in Minas Gerais.29 In primary health care (PHC), Dutra et al30 (2014) reached a prevalence of 13.63% of CKD (GFR < 60 mL/min/1.73 m2 from only one serum creatinine evaluation) in 8223 elderly, similarly to the prevalence of 15.4% found by Comini et al24 (2020) in hypertensive and diabetic patients also at the PHC level. It is important to highlight that the higher prevalence value found in our study is justified by the fact that we worked with a sample of patients with AH and/or DM, in the age group predominantly over 60 years, consisting of the main risk factors for CKD.
It is worth mentioning that AH31 and DM32 are considered as the main causes of CKD. AH was present in 30 of the 31 CKD patients evaluated. Hypertensive nephropathy involves loss of nephrons, endotelial injury with remodeling of the arteries, sympathetic hyperactivity and the renal angiotensin-aldosterone system (RAAS) with hydrosaline retention, increasing peripheral vascular resistance and, consequently, systemic blood pressure. In turn, AH leads to the remodeling of the aferente arteriole with loss of its regulatory capacity resulting in glomerular overload, nephrosclerosis and functional loss of the kidney.13
In this research, about 1 in 2 CKD patients was diabetic. In diabetic nephropathy, chronic hyperglycemia and forms of advanced glycation final products, stimulate growth factors, release of reactive oxygen species and pro-inflammatory substances.14 Consequently, a process of glomerular hyperfiltration increases in conjunction with parenchymal lesion (mesangial extracellular matrix deposition with diffuse or nodular expansion, thickening of the glomerular basal membrane, tubular atrophy and hyaline arteriolosclerosis), albuminuria and AH with interstitial fibrosis and glomerulosclerosis.14
Regarding the association of UA with CKD, we found that the increase of 1 mg/dL of UA increases by 65% the chance of the individual presenting CKD. In fact, UA is an organic molecule of mostly urinary excretion related to the function of the kidneys. It is freely filtered in the glomeruli, reabsorbed and secreted in the proximal convoluted tubule and only 10% of the filtered UA is finally excreted by the kidneys.33 Numerous studies have collectively built a body of evidence that, while not yet fully robust, challenges the notion of UA as a mere epiphenomenon of glomerular dysfunction.34 35,36
In this context, epidemiological and intervention studies suggest the high serum levels of UA as a cause of both the development of CKD and its clinical progression.37 With current knowledge it is believed that UA induces oxidative stress and endothelial dysfunction. Moreover, it acts as an alarm signal, engaging in intricate interactions with the innate immune system through cytosolic receptor recognition, initiation of cascading intracellular signals, and the release of pro-inflammatory cytokines.
This process influences the adaptive response and vice versa, culminating in sterile inflammation. This chronic inflammation holds the potential to develop into parenchymal fibrosis and tubulointerstitial atrophy, offering an explanatory framework for the onset of CKD.19,38,39,40,41
Our study has strengths to highlight. First, the high prevalence finding emphasizes the importance of including CKD in health planning, action and management in the context of PHC. Moreover, it describes a demographic, clinical, behavioral and biochemical profile of the population with CKD by expanding knowledge about its determinants and health conditions, that are relevant to guide disease prevention, promotion and rehabilitation activities in health. Second, a positive association between UA and CKD strengthens the existence of a possible interaction in CKD development and progression, demanding future research. Finally, the serum UA is a low-cost, wide-availability, minimally invasive, reproducible and comparable test over time. Confirming its potential as a predictor of CKD, it could result in its integration into routine screening tests for target organ injuries in hypertensive and/or diabetic patients. This would enable early diagnostic and therapeutic interventions, effectively reducing morbidity, mortality, healthcare expenses associated with hospitalizations and replacement therapies, and ultimately enhancing the prognosis and quality of life for affected individuals.
On the other hand, the present study also has some limitations. Firstly, it is a cross-sectional study, thus we cannot infer the causality from these results, but formulate hypotheses that should be confirmed on longitudinal, controlled and randomized studies. Secondly, the standardization of albuminuria measurement in the 24-hour urine test posed challenges due to technical difficulties in individual collection of the examination and variations in analysis methods used by laboratories within the municipality’s Health Care Network. As a result, we excluded this parameter from our investigations.
Considering that the presence of albuminuria in a 24-hour sample is one of the criteria for the diagnosis of CKD,26 the prevalence of CKD may have been underestimated. Third, there was no adjustment for the use of hypouricemic drugs as a confounding variable, which may have underestimated plasma levels of UA in patients with hyperuricemia.
CONCLUSION
Our study revealed a CKD prevalence of 17% among elderly participants, with important risk factors as hypertension and diabetes. Notably, each 1 mg/dL increase in UA corresponded to a 65% higher likelihood of an individual having CKD. Further studies are crucial to confirm the association, and define the role of UA in the pathophysiology of CKD. If proven, UA can be an accessible and useful tool in the prevention and management of CKD in PHC.















