Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.21 no.2 Lisboa abr. 2014
https://doi.org/10.1016/j.jpg.2013.10.004
IMAGES IN GASTROENTEROLOGY AND HEPATOLOGY
Hiatal hernia involving pancreas body: An unusual finding
Hernia do hiato com envolvimento do pancreas: uma situação pouco frequente
Joana Carvalheiro∗, Sofia Mendes, Carlos Sofia
Department of Gastroenterology, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
*Corresponding author
Type IV paraesophageal hiatal hernia (PEHH) is characterized by a large defect in the diaphragmatic hiatus that allows other organs, besides stomach, such as the colon, pancreas, spleen, or small intestine to herniate into the thorax.1 Herniation of the pancreas through a gastroesophageal hiatus is a rare condition, and only a few cases have been reported in the literature. We describe the case of a patient with herniation of the pancreatic body.
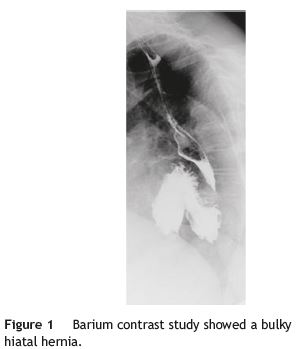
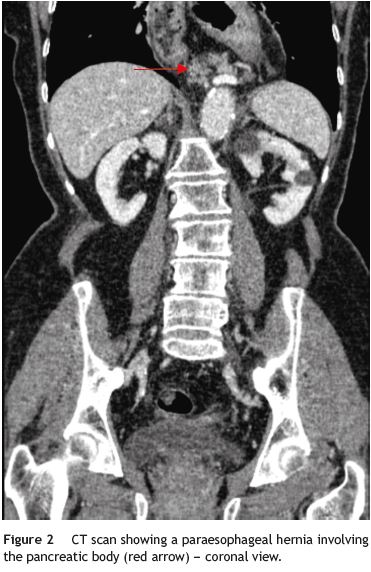
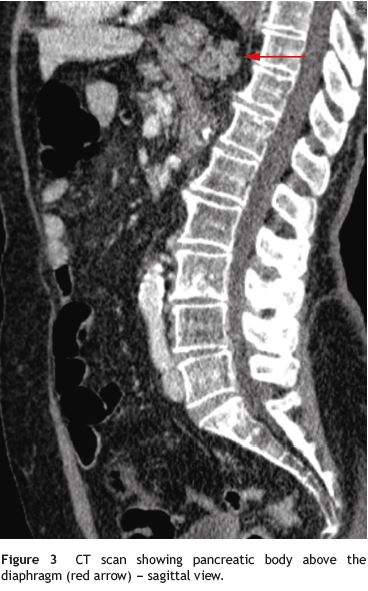
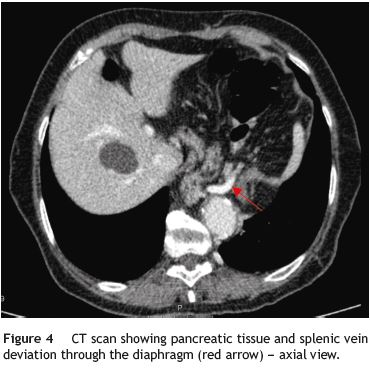
A 79-year-old woman was referred to our department complaining of postprandial epigastric pain often radiating to the back, associated to early satiety, nausea and heartburn. She had a passed medical history of arterial hypertension and dyslipidemia. Aside from mild epigastric and left hipocondrial tenderness on abdominal examination, her physical examination was normal. Upper gastrointestinal endoscopy and barium contrast study showed a bulky hiatal hernia (Fig. 1). No significative changes were seen on laboratorial or ultrasound investigation, although pâncreas could not be properly visualized due to intense aerocolia. The research proceeded with an abdominal CT which enabled intrathoracic location of a great proportion of the stomach along with the body and part of the tail of the pâncreas (Figs. 1, 2, 3 e 4). The patient was then submitted to surgical treatment. Reduction was easily effected, and the opening in the diaphragm was repaired. Recovery was uneventful and the patient became symptoms-free.
Four types of hernias have been described in the literature. Type I, also called sliding hernias, account for up to 95% of all hiatal hernias and occur when the GE junction migrates into the posterior mediastinum through the hiatus. Type II occurs when the fundus herniates alongside the esophagus through the hiatus, remaining the GE junction normally positioned. Type III is a combination of types I and II hernias with a displaced GE junction as well as stomach protruding through the hiatus into the thorax Type IV paraesophageal hernias are very rare, representing 5-7% of all PEHH and result from a combination of increased intra-abdominal pressure and a large hiatal defect. The colon, particularly the splenic flexure, is the most common organ that follows the stomach into the chest. Other common organs include loops of the small bowel and omentum. It is extraordinarily rare for the pâncreas to herniate in paraesophageal hernias.2 Patients may be asymptomatic or present any of the typical or atypical symptoms seen in the other three hernia types.3 Symptomatic PEHH in operable patients should be repaired. The underlying surgical principles for successful repair include reduction of hernia contents, removal of the hérnia sac, closure of the hiatal defect, and an antireflux procedure.4
References
1. Kahrilas P, Kim H, Pandolfino J. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601-16. [ Links ]
2. Coughlin M, Fanous M, Velanovich V. Herniated pancreatic body within a paraesophageal hernia. World J Gastrointest Surg. 2011;3:29-30. [ Links ]
3. Scott Davis S. Current controversies in paraesophageal hérnia repair. Surg Clin North Am. 2008;88:959-78. [ Links ]
4. Schieman C, Grondin SC. Paraesophageal hernia: clinical presentation, evaluation, and management controversies. Thorac Surg Clin. 2009;19:473-84. [ Links ]
*Corresponding author
E-mail address: joanameloc@gmail.com (J. Carvalheiro).
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interest
The authors have no conflicts of interest to declare.
Received 3 June 2013; accepted 16 October 2013













