Introduction
Esophagogastroduodenoscopy (EGD) is considered the gold standard in the investigation of gastrointestinal symptoms. A high-quality EGD is extremely important in the diagnosis and even treatment of early cancers [1].
In contrast to colonoscopy, for which there are well-defined quality benchmarks, EGD lacked criteria that would effectively standardize the quality of this exam by reducing the variability, which is still considerable, be-tween different centers and performers. At the end of 2015, the European Society of Gastrointestinal Endoscopy (ESGE) and the United European Gastroenterology (UEG) gathered a task force aiming to develop standards on quality in EGD, and by the end of 2016, new guidelines for performing high-quality endoscopy were published in the journal Endoscopy and in the United European Gastroenterology Journal [2].
The new ESGE/UEG guideline does not mention patient sedation; therefore, it is assumed that this would not be essential in the achievement and compliance of all the stipulated quality parameters. In the current literature, recommendations regarding EGD sedation practices are highly variable, and there is a lack of evidence to support the potential implications with regard to safety and efficiency of the procedure [3]. The purpose of this prospective study was to determine whether the use of deep sedation, administered by an anesthesiologist, influences the fulfillment of the quality criteria proposed by ESGE/UEG for EGD.
Materials and Methods
This was a cross-sectional study, with a prospective enrollment, that considered for inclusion consecutive patients referred for diagnostic or surveillance EGD in the Gastroenterology Department of a tertiary referral hospital, Centro Hospitalar e Universitário de Coimbra, Portugal. Inclusion criteria included patients who were 18 years of age or older, had completed the required fasting time (6 h for solids and 2 h for clear liquids), and were indicated to undergo EGD only with diagnostic or surveillance objectives.
Patients non-compliant with fasting or presenting food residues in the stomach were excluded. Since this was a study that focused on the diagnostic use of EGD, all patients whose indication for the examination included the performance of endoscopic therapy or exams performed in an emergency context were excluded. The patient’s refusal to participate in the study was also an immediate exclusion criterion. Another exclusion criterion was the history of previous esophageal, gastric, or duodenal surgery.
When inclusion criteria were fulfilled and there was no exclu-sion criterion, the patient was allocated to one of the following groups, according to the assistant physician’s request that considered the patient’s preferences/demands: EGD without sedation or EGD with sedation. The investigators had no interference in this decision process.
A selection bias is related to the fact that not all EGDs at the center were included. Only EGDs performed by the 3 endoscopists, who were aware of quality evaluation and were equally distributed in the sedation and non-sedation suites, were included.
Regarding sedation, its depth varies in a continuous spectrum, and it can be graduated into 4 levels: minimal, moderate, deep, and general anesthesia. To assess the true impact of sedation in EGD, deep sedation with propofol administered by an anesthesiologist was the approach used in the sedation group. All procedures were performed by three gastroenterologists with extensive experience in endoscopy, and the patients were monitored according to standard parameters (pulse oximeter, heart rate, blood pressure, capnography and electrocardiogram).
In each case, two questionnaires were applied. One, for the researcher, focused on evaluating the compliance of the quality parameters proposed by ESGE/UEG. The other questionnaire was designed to allow the assessment of the degree of the patient’s satisfaction regarding the exam, on a numerical scale of 0-10 (0 - not at all satisfied; 10 - extremely satisfied).
Sample size calculation was performed. Assuming that it will be possible to meet the quality criteria in 70% of endoscopies performed without sedation and in 90% of cases performed with seda-tion, the sample size, calculated for a 95% confidence interval and 10% margin of error, was 92 patients in each group. So, inclusion for each group stopped once this number was achieved.
Data was recorded between July 2019 and March 2021. All statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS, Version 23.0).
This study was approved by the Ethics Committee of the Faculty of Medicine, University of Coimbra, Coimbra, Portugal, on June 24, 2019. The privacy of the research patients participating was guaranteed, and the protocols followed the tenets of the Declaration of Helsinki.
Results
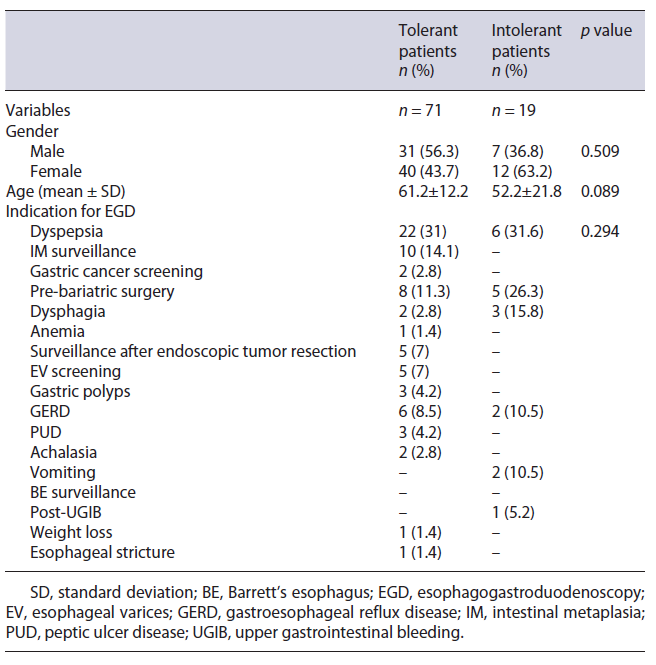
A total of 184 patients were included in the study, 92 of whom underwent EGD without sedation and the remaining 92 with deep sedation. Table 1 shows the sample characterization. Females predominated (62.5%) in both groups, representing 66.3% (n = 61) and 58.7% (n = 54) of the cases with and without sedation, respectively. The mean age of the sample was 61.3 ± 16.9 years (62.7 ± 15.9 in the anesthetic group vs. 59.9 ± 17.8 in the non-sedation group; p = 0.231). The mean age of intolerant patients was lower, compared with patients who underwent complete examination, but this difference was not statistically significant (52.2 vs. 61.2 years, p = 0.089) (Table 2).
In the group of patients who underwent EGD under sedation, the majority had an ASA II classification (67.4%), followed by ASA III (18.5%) and ASA I (14.1%), respectively. In this studied sample, the predominant indications for performing an EGD were dyspepsia (n = 66; 35.9%), intestinal metaplasia surveillance (n = 18; 9.8%), and gastroesophageal reflux disease (n = 16; 8.7%). Other relevant indications were pre-bariatric surgery study (n = 14; 7.6%), dysphagia (n = 11; 6%), anemia (n = 9; 4.9%), and surveillance after endoscopic resection of esophageal, gastric or duodenal lesions (n = 7; 3.8%). These data are shown in Table 1. According to the results obtained, the exam indication influenced the request, or not, for deep sedation (p = 0.003). This association is mainly due to exams whose indications were pre-bariatric surgery (13 exams without sedation and only 1 with sedation), surveillance after neoplastic resection (all procedures without sedation), anemia (only 1 exam was performed without sedation and the remaining 8 with sedation), and gastric cancer screening (only 1 was performed without sedation and the remaining 7 with sedation).
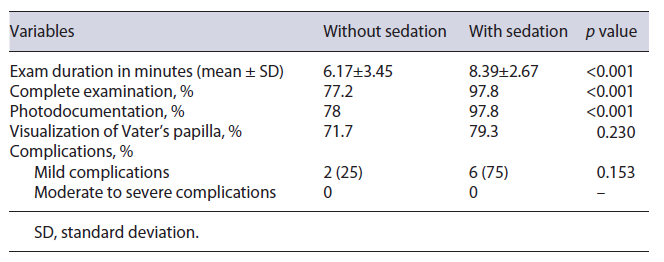
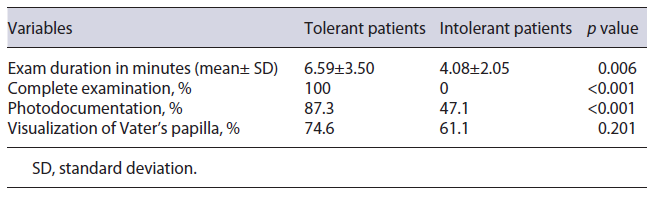
Analyzing the impact of sedation on compliance with quality criteria published in the ESGE/UEG guideline, it was found that there was a statistically significant association (p < 0.001) between the use of sedation and the ability to perform a complete EGD, represented by the following variables: complete examination (77.2% without sedation vs. 97.8% with sedation); photodocumentation of the main anatomical sites (78% vs. 97.8%) (Table 3). The main reason for not performing a complete exam was patient intolerance (82.6% of the non-complete exams), which occurred exclusively in non-anesthetized patients. The impact of tolerance in the no sedation group on high-quality EGD performance is shown in Table 4. In the sedation group, only 2 patients (2.2%) did not undergo a complete examination because of hemodynamic instability, which was reversed with supportive measures. Intolerance in non-sedated patients justified the short inspection time (6.17 ± 3.45 vs. 8.39 ± 2.67 min) and the low percentage of EGD lasting more than 7 min (28%), as shown in Tables 3 and 5, respectively.
Table 5 Impact of sedation on the diagnosis of gastric and esophageal lesions and on surveillance of preneoplastic gastric lesions
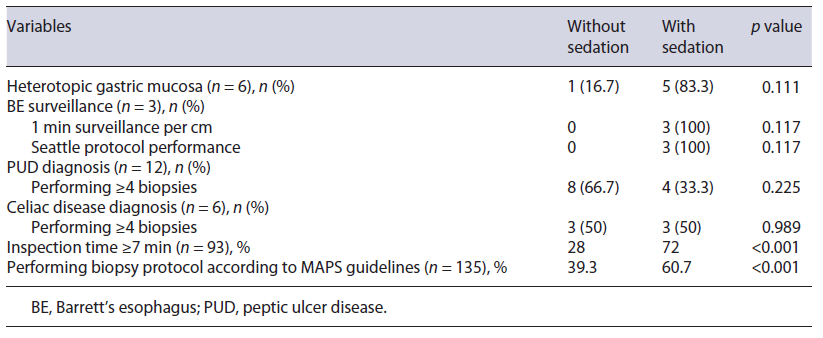
Additionally, a comparison of tolerant and intolerant patients in the no sedation group was performed, and it is shown in Tables 2 and 4. Ninety was the total number of patients included in this analysis. Two patients were excluded since the reason for an incomplete examination was not related to tolerance but to the occurrence of complications. None of the demographic characteristics or indications to perform the exam influenced the tolerance in the no sedation group.
Regarding the surveillance of premalignant gastric lesions (Table 5), it was shown that the use of sedation favors a minimum inspection time of 7 min (72% vs. 28%; p < 0.001) and the performance of adequate biopsy protocol according to MAPS guidelines (60.7% vs. 39.3%; p < 0.001) [4]. Assessing the impact of sedation on performing, at least 4, biopsies, when indicated, in the context of celiac disease and/or gastric ulcer, we found that there is no statistically significant association. However, the small number of cases of gastric ulcer (n = 12) and celiac disease (n = 6) may weaken this result (Table 5).
In relation to gastric heterotopia in the proximal esophagus, it was shown that the use of sedation favors it, although without a statistically significant difference (p = 0.111). In the group without sedation, the diagnosis of gastric heterotopia was made in 1.1% of the patients (n = 1), compared with 5.4% (n = 5) in the group with sedation (Table 5).
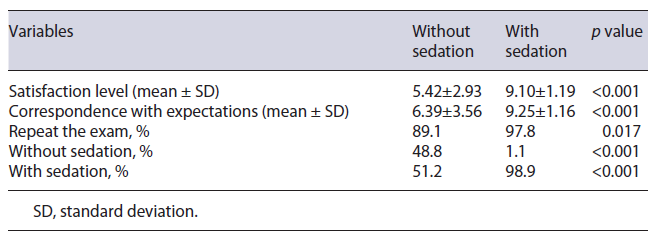
In the patient experience area, the use of sedation pro-vided an average level of satisfaction considerably higher than in the group without sedation (9.1 ± 1.2 vs. 5.4 ± 2.9) and allowed a greater correspondence with expectations (9.3 ± 1.2 vs. 6.4 ± 3.6). The use of sedation also influenced the predisposition to repeat the exam in the future: 98.9% of the patients who underwent EGD with sedation would repeat the exam with sedation. On the other hand, less than half of patients who underwent EGD without sedation (48.8%) would accept to perform an EGD under the same conditions (Table 6).
The high satisfaction reported by patients who underwent EGD under sedation was congruent with the greater predisposition of these patients (98.9%) to repeat the exam, as shown in Table 6. Globally, reported complications were rare (n = 8; 4.4%) and classified as mild, according to the common terminology criteria for adverse events. There was no statistical association (p = 0.153) between the use of sedation and the occurrence of complications associated with EGD (Table 3).
Discussion
The quality of health service is established by its ability to provide the best clinical outcomes. Therefore, high-quality endoscopy must be able to recognize or exclude correct and relevant diagnoses [5]. In the upper gastrointestinal tract, it is acknowledged that the detection rate of premalignant lesions is often suboptimal, in most cases due to a failure in the execution of the technique [6, 7]. At the end of 2016, a new guideline for performing a high-quality endoscopy was published in ESGE/UEG journals. [2] The ESGE/UEG guideline describes major and minor quality criteria (performance measures), which are related to pre-, per-, and post-procedure aspects. The first and the last are relatively restricted and do not depend, to a great extent, on the performer or the patient, as they are standardized. On the other hand, the per-procedural criteria require not only improved care by the gastroenterologist but also a high level of patient cooperation. The prolonged time proposed for each endoscopy often generates intolerance in non-sedated patients, frequently leading to premature interruption of the exam. The tolerance level of patients varies widely, and it is recognized that some patients need to be sedated to obtain a high-quality EGD [8-10]. In current literature, recommendations regarding sedation practices in EGD are highly variable, and there is a lack of evidence supporting the potential implications for the safety and efficiency of the procedure [3]. The ASGE suggests the use of deep sedation when it is expected to favor comfort and safety for the patient, as well as the complete performance and efficiency of the procedure. However, this is a recommen-dation based on low-quality evidence, implying the need for additional studies [11].
In the present study, a positive link between the use of deep sedation and the applicability of per-procedural quality criteria in the domains described by the ESGE/UEG is indorsed. After a thorough analysis of the different parameters, we found that patients intolerant to EGD were younger than those that had a complete examination. Similar data were observed in a study that highlighted a greater tolerance in the older population (>75 years) to perform the exam without sedation [12]. These data could be taken into account when selecting potential candidates for EGD under sedation.
A longer inspection time reflects a more complete examination and is associated with greater diagnostic accuracy, therefore being a major criterion [2]. A high-quality EGD should last at least 7 min from intubation to extubation [2, 10, 13]. In this study, we concluded that the mean duration in the group without sedation was suboptimal (6.17 min), mainly associated with early interruption due to patient intolerance, which was translated into a lower rate of exam completion. Less than a third of the patients undergoing EGD without sedation benefited from a minimum gastric inspection time of 7 min, which raises concern about the lower effectiveness of the examination and possible underdiagnosis of pre-neoplastic or neoplastic lesions [6, 14, 15]. This is particularly relevant since procedures lasting at least 7 min are capable of detecting three times more dysplastic lesions and gastric cancer [2].
Appropriate photographic documentation is also a major criterion in the ESGE/UEG guideline. The present study demonstrated that this key quality criterion benefits significantly from the use of sedation, which can be explained by the reduction in artifacts associated with patient movement.
Population with atrophic gastritis and intestinal metaplasia has a higher prevalence of gastric cancer [15]. These pre-neoplastic lesions are often distributed irregularly in the stomach; consequently, their correct diagnosis and staging require biopsies [16]. The EGD performed under sedation eased the performance of biopsies, promoting higher diagnostic effectiveness. Early detection of gastric cancer allows for less invasive and more effective treatment, being an important prognostic factor and reducing the economic impact associated with the treatment of more advanced stages [17].
Regarding the impact of sedation on Barrett’s esophagus (BE) surveillance, there was a predominance (98.4%) of neutral responses: “NA” (not applied). Although the number of cases of BE surveillance is limited, the Seattle protocol was only applied to patients under sedation. This protocol’s performance is a major quality criterion in the field of pathology management. It ensures maximum diagnostic sensitivity, making possible the early detection of dysplasia and esophageal adenocarcinoma, with a positive impact on mortality rate (the 5-year survival rate of invasive esophageal adenocarcinoma is less than 20%) [18]. Despite the proven value of the Seattle protocol, shown in the recommendations of many societies, there is still insufficient compliance to its application (up to 20% non-adherence) [19]. The present study indicates that the risks associated with the ineffectiveness of performing biopsies which allows a safe BE surveillance probably justifies the use of deep sedation in these patients. Even if the results are not statistically significant, due to the reduced number of cases, it is fair to infer that deep sedation ensures more adequate BE surveillance (Table 5).
Overall, in agreement with the literature, it was demonstrated that patients undergoing sedation had better experience in performing EGD [3, 11, 12, 20, 21]. In the domain of experience for the patient, the use of sedation provided an average level of satisfaction considerably higher than the group without sedation, allowing a greater correspondence with expectations and predisposition to repeat the exam. Thus, it is expected that in patients enrolled in a surveillance program which requires periodic EGD, the use of deep sedation can determine a higher compliance. This attitude allows for an early diagnosis of certain lesions, promoting a better outcome for patients and reducing health costs in the long term. It is described that deep sedation is more satisfactory for the patient and increases the predisposition to repeat the exam [11, 20, 21]. Given the difficulty of having an anesthesiologist present for most of the exams performed, it would be interesting to assess, in a future study, whether the fulfillment of EGD quality criteria is compromised when using minimal or moderate sedation in comparison to deep sedation.
At a financial level, performing an exam with sedation outweighs the costs associated with the same exam without sedation. The use of sedation entails costs related to human resources, hospital supplies, and drugs. Nevertheless, it is recommended that incomplete exams due to patient intolerance be repeated under sedation [10, 12]. Therefore, it can be expected that avoiding the use of sedation in EGD may imply the cumulative cost of two exams, greater inconvenience for the patient, and exposure to iatrogenic risks, consequently surpassing the initial costs associated with performing an EGD with sedation. A cost-effectiveness analysis should be performed to determine which would be the more effective approach: EGD with no sedation, gastroenterologist-administered sedation on demand and in specific groups, or deep sedation by an anesthesiologist in selected patients.
Monitoring complications is an important criterion to establish the safety of EGD [2]. In the present study, the complication rate was low (4.4%) and showed no significant relationship with the use of sedation. Multiple studies have been comparing propofol sedation to traditional sedation, and they have confirmed the safety of deep sedation, which is considered similar to light and moderate sedation [11, 20-22].
Given the difficulties of performing all EGD under sedation, it is acceptable to select patients or outline a user profile that will certainly need to benefit from sedation to perform a quality EGD. Therefore, with this study, we can conclude that the request for an examination with deep sedation, especially in young patients or in those who must be included in a screening or surveillance program, should be considered.
This study has some limitations. The first one is the lack of randomization. In fact, patients were previously allocated to sedation or no sedation, and the investigators had no interference in such process. That can be assumed as a major bias, but on the other hand, one can assume that if a patient accepts to perform EGD without sedation, he/she might be more motivated to do so. On the contrary, if a patient requests sedation, he/she is not keen to do such procedure without sedation, and if obliged, his/her compliance would probably be worse. Therefore, it is not ethical to randomize patients to sedation or no sedation if they have previously chosen the opposite with their assistant physician. The second limitation of this study, which is tightly linked to the first one, is the presence of some differences in indications. However, when we analyze what was necessary to achieve the quality objectives such as procedure time, photodocumentation, performance of biopsies, and completeness of the exam, there is a clear difference between the procedures with and without sedation, independently of indication. Also, many patients submitted to endoscopy without anesthesiologist support stated that they would repeat the procedure only if sedation was offered to them. The third limitation was the strict inclusion and exclusion criteria. This study was performed in a tertiary referral hospital with many patients being referred for EGD with therapeutic objectives. Additionally, there is limited access to anesthesiologist-administered sedation that justifies the period necessary to achieve the desired number of patients in each group. It is also important to mention that not all EGDs at the center were included and only EGDs performed by 3 endoscopists who were equally distributed in the sedation and non-sedation suites. This can be assumed as a selection bias. Finally, as we previously stated, there are different levels of sedation, and minimal or moderate sedation, administered by gastroenterologists, was not considered in this study.
Conclusion
The present study seems to demonstrate that the use of deep sedation can influence the fulfillment of a high-quality EGD, in accordance with the ESGE/UEG criteria. The use of sedation suppressed the patient’s intolerance, promoting a higher quality EGD. The administration of sedation was safe and was not associated with an increase in adverse event rates. Patients’ satisfaction was also considerably higher after EGD under deep sedation.
We conclude that the use of deep sedation in clinical practice should be considered in patients undergoing EGD, to ensure a high-quality examination. A comparison with minimal or moderate sedation, administered by gastroenterologists, is needed.