Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. v.24 n.2 Dois Portos 2009
Biological diversity preservation as a challenge - the role of microbiological Cork colonisation a short review
A diversidade biológica um desafio do presente - papel da colonização microbiológica da Cortiça
M. V. San Romão 1,2
1 L-Instituto Ncional de Investigação Agrária - Ex Estação Vitivinícola Nacional. Quinta de Almoínha. 2565-901 DOIS PORTOS. Portugal
2 Instituto de Biologia Experimental e Tecnológica/Instituto de Tecnologia química e Biológica - Universidade Nova de Lisboa.
Av da República. Apt 12. 2781-901 OEIRAS. Portugal
(Manuscrito recebido em 17.11.09 . Aceite para publicação em 07.12.09)
SUMMARY
Micro organisms are important sources of knowledge being of critical importance to the sustainability of life on Earth. The biodiversity preservation either of animals, plants or microorganisms and the use of alternative ways to explore their respective potential richness in a balanced manner implies deeper research in these areas covering the different required subjects. Changing both governmental and local perceptions about the obligation to preserve biodiversity, including microbial diversity, requires the demonstration that the sustainable use of biodiversity has positive economic value. The studies summarized on this paper respecting to cork microbial colonisation, tried to illustrate the relationship between microbes, effects on ecosystems behaviour, technological implications on society activities and subsequent economic impact. The requirement of increased efforts in the preservation of the oak forest as a sustainable environment namely due to the carbon assimilating and retention capacity of these systems and also the requisite of improved research efforts aiming to better understand the true role of cork fungal colonisation and to define suitable strategies to preserve cork stoppers use to seal wine bottles are also discussed. It is stress the fact that the increase in the market of alternative wine stoppers will reduce the economic value of cork forest therefore leading a finally loss of one of the best and most valuable examples of a human-nature balanced system.
Palavras-chave: Ecologia microbiana; diversidade microbiana; sustentatibilidade da Terra; microbiota da cortiça; rolha de cortiça
RESUMO
Os microrganismos são fontes importantes de conhecimento sendo críticos para a manutenção da sustentabilidade da vida na Terra. A preservação da biodiversidade de plantas, animais ou microrganismos e o uso de tecnologias alternativas de exploração do seu potencial de forma equilibrada implica um conhecimento profundo e investigação em diferentes áreas contemplando os necessários aspectos específicos. Alterar a percepção da responsabilidade e obrigação de preservar a biodiversidade, incluindo a diverdade microbiana, tanto a nível governamental como local implica a demonstração de que o uso sustentado da biodiversidade se traduz num efectivo valor económico. A revisão dos conhecimentos sobre a colonização microbiana da cortiça apresentada neste trabalho pretende ilustrar um exemplo de relações entre microrganismos, respectivos efeitos no ecosistema, implicações tecnológicas em actividades da sociedade e subsequente impacto económico. É realçada a necessidade de ser reforçado o investimento na preservação do montado, um exemplo de floresta sustentada, com particular impacto na retencão de CO2. Também é realçada a necessidade de investimento acrecido em investigação por forma a aprofundar o estudo do papel da colonização fúngica da cortiça e definição de estratégias adequadas a adoptar por forma a preservar o uso da rolha de cortiça como vedante previligiado de vinhos de qualidade. De realçar o facto de o aumento no uso de rolhas alternativas às de cortiça, se reflectirá inevitavelmente no decréscimo do valor económico da cortiça e a médio prazo na destruição de um dos sistemas naturais mais sustentados.
Keywords: Microbial ecology; microbial diversity; Earth sustainability; cork mycrobiota; cork stopper
Microbial ecology and diversity
Microbial ecology examines the micro organisms present in ecosystems and also the nature and extent of their metabolic activities. Microbial diversity cover the spectrum of variability among all types of micro organisms (bacteria, fungi, viruses) in the natural world, unfortunately a large amount disturbed by human intervention. As a consequence, microbial diversity is an invisible resource that justifies greater attention.
Micro organisms are the oldest, most diverse and most abundant forms of life on Earth. However, the identity, physiology and interactions of the vast majority of these microbes, as well as the processes they mediate in the environment, remain unknown or poorly understood. Micro organisms are essential for the Earth to function and the knowledge of microbial diversity is essential to understand evolution. They play many roles both on land and in water, including being the first to colonize and ameliorate effects of naturally occurring and man-made disturbed environments (Colwell, 1997).
Studies of microbial diversity are difficult remaining one of the greatest challenges in microbial ecology. Samples of a distinct ecosystem are hardly considered as large enough to be representative of the respective ecosystem under study. Moreover, sampling is dependent on available budgets and the technologies and assessment required to get a realistic representation of the diversity is not enough valued. Also factors like ecosystem environmental conditions, microbial competition for nutrients, microbes growth rates, microbial activities, need to be taken into account (Konopa, 2006). Curtis and Sloan (2005) comparing the study of microbial diversity to exploring outer space, referred that Astronomers have judiciously inferred the population of celestial objects by mathematical assumption. The authors pointed to the existence of more than 1016 prokaryotes in a ton of soil compared to a simple 1011 stars in our galaxy. As a result, during the most recent years, microbiologists began to apply to microbial ecological studies some tools, like mathematical models, trying the simulation of natural conditions in order to overcome some of the most important constraints inherent to this research area. However, this approach continues to be considered exploratory and, the definition of satisfactory data for such surveys is yet difficult to be found (Sloan et al, 2007). Although this methodology can also be considered speculative it allows microbial ecologist to move into a new phase in which the estimation and description of microbial diversity becomes a rational and planned activity. Following this trend Quince et al (2008) applied a statistical method to large gene sequence libraries to estimate soil and ocean diversity. Using taxaabundance distributions they purposed a plan and developed the required methods and strategies in order to estimate the diversity in the ecosystems as well as the degree of sampling required to index that diversity. The authors concluded that the rates of recovery of new microbial taxa in very large samples suggest that many more taxa remain to be discovered. These works are being initiate a new phase in the exploration of the microbial world.
Microrganisms as stability and functioning agents of ecosystems
As stated before, microorganisms are important sources of knowledge about the strategies and limits of life, being of critical importance to the sustainability of life on our planet. The unexploited diversity of microorganisms is a resource for new genes and organisms of high value to biotechnology (Bertin et al., 2008).
Microbes developed the basic types of metabolism and a wide range of activities, which allow them to colonize all ecosystems. As they are also the starting point of global cycles of elements, micro organisms contribute significantly to the stability and functioning of ecosystems, which can be endangered by man-made disturbances including those originating from biotechnological applications (Carney and Matson, 2005). Thus, the sustenance of the environment requires a thoughtful management of microorganisms (Tscharntke et al., 2007). Microbial diversity can be regarded on one hand as a problem, for example due to the large variety of micro organisms causing diseases and on the other hand as a solution due to the rich biotechnological potential including diseases control (Rook and Brunet, 2005; Mazmanian et al., 2008). For example the diversity, biological activity and secondary metabolite production of fungi associated with marine sponges were investigated in order to assess the potential of these fungi for the production of novel biologically active secondary metabolites as sponges are a host of diverse microbial communities and are also a very potent source of biologically active natural products. However, the exploitation of the chemical diversity of sponges and their microbial symbionts is problematic although metagenomics associated to new techniques adequate to microbial exploitation appears as a sustainable and cost-effective solution (Holler et al., 2000; Kennedy, 2007). Another example are the relationships between soil microbial communities and ecosystem functions which are growing through increasing recognition of the key roles that micro organisms play in a variety of ecosystems. The study of such associations is essential to assess their significance for the survival and performance of plants and animals. This kind of interactions between microorganisms and hosts represents a classical ecological relationship. The associations of microorganisms with hosts and other habitats are critical determinants for many topics relevant for mankind such as the quality of the environment, global change or the decline of food (Welbaum et al., 2004). Diversity patterns of microorganisms can be used for monitoring and predicting environmental change (Thuiller, 2007).
Microbial communities are excellent models for understanding biological interactions and evolutionary history as microorganisms play an imperative role in conservation and restoration biology of higher organisms. Interactions among micro organisms and between micro organisms and their physical environment are complex and reflect a network of integrated physiological responses and regulatory mechanisms. Many organisms are known to modify the environment in order to construct an adequate niche where a natural selection can take place (Brown et al., 2009). Some of the secondary metabolites produced by microorganisms can act as info chemicals inside the ecosystem. Many of these are volatile and volatile organic compounds (VOCs) that have been identified in soil atmospheres and that have been related to community structure and function (McNeal and Herbert, 2009). VOC profiles produced by microorganisms have been shown to be consistent, relating to cultural conditions, environment input, and so to population and function dynamics. Interactions via these compounds result in functional responses by the organisms involved, giving selective advantage to some community members. VOC-mediated positive, negative or neutral interactions can occur between a very wide range of soil bacteria and fungi. These effects include both stimulation and inhibition of growth, and enzyme production being only an example of the different surviving strategies used by the biological communities.
The concept behind the idea of ecological strategies is that different environments select for different character in the organisms that live in them. Those strategies may involve complex social interactions that occur both within and between species and can be either competitive or cooperative (Velicer 2003; West et al., 2006). Although cooperative systems can persist in nature despite the potential for exploitation by non co-operators, it is often observed that small changes in population demography can influence the balance of selective forces for or against cooperation. An example of a cooperative interaction is the existing one between leguminous plants and the rhizobial bacteria that fix nitrogen within the root nodules of the host plant. Masson-Boivin et al.(2009) have referred that a variety of mechanisms appeared to be involved in this symbiosis pointing to the requirement of deeper studies in order to their elucidation and biochemical characterization. On another hand, microbial competition for the existing resources available appears as a powerful alternative to the community ecological strategy and can be defined as the negative effects which one organism has upon another by consuming, or controlling access, to a resource that is limited in availability. Competition for nutrients and other resources is the most common interaction that occurs between two or more microbial species inhabiting the same environment (Widen, 1997, Gaki et al. 2009). However the competition concept appears in the literature as being controversial (Cooper, 1993, Miller et al., 2005). Part of the reason of this controversy is due to the difficulties in demonstrating that competition actually exists, in both experimental systems and natural communities.
A lack of conceptual and theoretical approaches to the studies of microbial survival strategies is claimed. The role of competition in determining the distribution of species and the structure of communities points to the major requirement of an ecological theory (Prosser et al., 2007). Although the fundamentals of evolutionary theory seem to be well understood, there is still a need to enlarge the theory applying it to specific microbiological cases in order to understand and explain the interactions between micro organisms and their environments, allowing microbial ecologists to draw together simple models and concepts using new approaches e.g. statistical models (Gans et al., 2005; Austin, 2007; Goldman and Brown, 2009). The nature of competition in determining the distribution of species and the structure of communities defines the major significance of competition theory. Moreover, diversity is characterised by a considerable pluralism of theories and policy paradigms (Lobry and Harmand, 2006; Sapp, 2007; Harmand et al., 2008).
Scheiner and Willing (2008) defined the seven fundamental principles necessary for a general theory of ecology: "the heterogeneous distribution of organisms, interactions of organisms, contingency, environmental heterogeneity, finite and heterogeneous resources, the mortality of organisms, and the evolutionary cause of ecological properties" and Fraser et al., (2009) have emphasized the need to combine genetic diversity and distinct ecological niches in an attempt to define species in a coherent and convincing approach establishing that evolutionary theory 'should be able to explain why species exist at all levels of the tree of life' and the need 'to be able to define species for practical applications in industry, agriculture, and medicine'. Moreover, soil ecologists, focussing on the differences between soils, emphasise the concept that soil ecology is an interdisciplinary field reflecting some of the most important advances in agronomy, ecosystem ecology, microbiology, and environmental science as well as the global climate change, biodiversity loss, and agricultural practices stressing that those different parameters need to be considered in such studies (Noah et al., 2009).
It should be emphasised that fungi play a major role in the function and dynamics of terrestrial ecosystems directly influencing the structure of plant, animal, and bacterial communities. There is an adequate amount of information in literature that clearly demonstrates that competition does occur in fungal communities and that it plays a role in their structuring (Maherali and Klironomos, 2007; Teste et al., 2009). Some interference mechanisms are well documented namely in soil or wood decay fungal communities (Heilmann-Clausen and Boddy, 2005). As stated above, one group of microbial organisms that plays a key role in the ecological function of many terrestrial ecosystems is mycorrhizal fungi (for a review see Pringle et al., 2009). Fungi can interfere with each one another depending on environmental conditions, that interference can involve the production of either volatile or soluble chemicals which may provoke the inhibition of growth or metabolic activity of one fungus by another one. The increasing interest of fungi either in agriculture and forestry should justify an increasing input on studying those issues as without it, the outcome of attempts to use fungi, either as bio-control agents or as beneficial symbiontes should be unpredictable. It will be necessary to have much more knowledge on the fungi activities in the different niches that they colonise (e.g. soil, plants, cork,…) developing a good understanding of the ecological strategies of each fungal community. Accordingly, it is necessary to identify the nature of the potential occurring competition phenomena and the fungi effects on the substrates available (Widden 1997; Toljander, et al., 2006). Without sound theoretical models it is not possible to overcome the lack of knowledge on fungi competition and interactions.
Economic value of biodiversity
To assess microbial biotechnological valuation it is crucial to understand how the goods and services provided by biodiversity are considered important; the social value endorsed to biodiversity and also the place that the individual character of confidentially takes on biodiversity, as it was ascribed by the Convention for Biological Diversity (http://www.cbd.int/biosafety/protocol.shtml).
According to Hamdallah Zedan (http://www.wfcc.info/ICCC7/keylectu.html), many direct economic values were derived from microbial diversity. Miyazaki (2006) stated that genetic resources, in particular microbial resources, are potentially valuable sources for future innovation of pharmaceutical and other industrial products. Micro organisms are used for food production and preservation, production of antibiotics and other medicines, as well as for much other industrial processes either in agriculture (e.g. increasing soil fertility) or in environment preservation (cleaning up of oil spills, waste water treatment) (Mohanty and Mukherji, 2008; Sánchez, 2009).
Actually, the exploitation of micro organisms to produce high added value products like biofuel had acquired increasing interest. Biomass represents an abundant renewable resource for the production of bioenergy and biomaterials, leading to a new manufacturing concept for converting renewable biomass to valuable fuels and other products (Ragauskas, et al., 2006; Stephanopoulos, 2007; Sánchez, 2009). These processes are a very important research field allowing either improvement of forests cleaning or production of alternative energy sources, aspects of increasing importance for the global planet sustainability.
The Convention on Biological Diversity (CBD) also states the principles for sustainable use of components of biological diversity. Greater understanding of the functioning of ecosystems combined with enhanced valuation techniques will have a higher impact on national conservation strategies. Benefits estimates can be important in showing that funds committed to conservation of microbial diversity and its use may be regarded as an investment that contributes to maintain and enhance the well being of communities and their economic income (Colwell, 1997; Kursar et al., 2006). According to the CBD principles, countries will be encouraged, in order to gain greater benefits from microbial resources, to increase their investment both in human and technological competences to isolate, preserve and characterize micro organisms and provide users with more value-added resources through scientific and technological education and training, scientific research, and technology transfer. The extent to which countries may possibly agree to the cost of conservation will depends on how fast and to what extent they will benefit materially from their microbial resources on a sustainable basis (Allen and Lord, 2004; Raffaelli, 2004). It can be important to show that the costs of proposed conservation programmes are justified. Such estimates may include the cost for the entire community if no action is taken to conserve biodiversity and use it in a sustainable way.
Using the cork micobiota as a case study, this review aims to illustrate the existing relationships between cork microbial communities and the processes they perform in this particular ecosystem as this can represent an example of fungi role on important sectors of human life: economic and technological exploitation.
Cork oak forestry covers approximately 2.7 million hectares of Portugal, Spain, Algeria, Morocco, Italy, Tunisia and France. These landscapes also support one of the highest levels of biodiversity among forest habitats, including globally endangered species such as the Iberian Lynx, the Iberian Imperial Eagle and the Barbary Deer (http://www.realcork.org/userfiles/File/Publicacoes/From%20the%20Cork%20Oak%20to%20Cork%20a%20sustainable%20system.pdf).
Cork is a natural and biodegradable material produced on the outer bark of the cork oak Quercus suber L. Its chemical and cellular structure makes cork a unique material for sealing still and sparkling wines namely due to its compressibility, resilience and waterproof properties. In the nineties some non-producing cork countries start to develop alternative synthetic sealing devices for wine bottles. They claimed for wine contamination by off-flavours (the so called 'cork taint') resulting from cork fungal metabolism. The main compound responsible by this defect was identified as the 2,4,6-trichloroanisole (TCA), resulting from the metabolism of polychlorophenolic compounds present in cork. This problem reflected in a large prejudice for cork stoppers and wine industries. As a consequence, both cork stopper manufacturing unities and research groups largely invested either in the upgrading of the manufacturing process it self or in deeper studies of the role of cork micota in the TCA production and cork stoppers properties. Strict rules are now defined to cork stoppers manufacturing process (http://www.celiege.com/Ingles/systecode/international_code/international_code.pdf) that are mandatory for cork stoppers manufacturing certification.
Cork tissue is a very porous material, 50% of the cork cell volume consists of air (the main reason for its particular mechanical properties) housing fungal spores (http://www.realcork.org/artigo.php?art=14), Pereira (2007). There is not adequate knowledge concerning the microbial behaviour in this special environment. Filamentous fungi and yeast were consistently detected in cork (Lefebvre et al., 1983; Lee and Simpson, 1993). Later on, the filamentous fungi isolated along the process of cork stopper manufacturing, were Chrysonilia sitophila, the most predominant one, followed by Penicillium glabrum, Mucor plumbeus and Trichoderma longibrachiatum (Danesh et al., 1997; Oliveira et al., 2003). The yeasts Candida famata, Sporidiobolus johnsonii and Rhodotorula glutinis were isolated only in early stages of cork processing. The changes in the cork as habitat, during the planks resting stage after boiling and the interactions between yeasts and other micro organisms are possible explanations for the limitation of yeast growth.
A partial inhibition of Rhodotorula glutinis growth was observed in presence of M. plumbeus and T. longibrachiatum (Figure 1). The other filamentous fungi (Aspergilus, P. glabrum and C. sitophila) appear as no producers of metabolites able of inhibiting yeasts growth under the used experimental conditions (results not published). This fact suggests that the antagonism mediated by specific or non-specific metabolites of microbial origin may constitute only a partial explanation for the growth pattern of filamentous fungi and yeasts throughout the cork processing.
As stated above C. sitophila is normally reported as the dominant fungus associated to the resting stage of cork slabs after boiling. Moreover, although some authors have considered this fungus as TCA producer (Alvarez-Rodriguez et al., 2002; Coque et al., 2003), it was observed by others that C. sitophila is unable to produce TCA during its growth in cork medium even in presence of poly-chlorophenolic compounds, and it appeared to be able to restrain the growth of TCA producing fungi (Silva Pereira et al,. 2000; Prak et al., 2007; Prat et al., 2009), this fact may be due to a strain dependent mechanism or used methodologies along the different studies. It can be stated that the responsibility of cork taint occurrence in wine cannot be attributed to all the fungi associated with cork during stopper manufacture. Additionally, Silva Pereira et al. (2000) reported that C. sitophila can tolerate higher pentachlorophenol (PCP) concentration than other fungi and suggested that the metabolic strategy used by C. sitophila to degrade pentachlorophenol resulted in a very high level of pentachlorophenol degradation without TCA production.
It is known that C. sitophila always dominate on cork slabs after boiling during the beginning of the resting stage and that on culture medium, when compared to other fungi isolated from cork slabs, C. sitophila presented the higher constant growth rate. Water activity (aw) necessary to C. sitophila growth is higher than 0.9 while Penicillium sp and Trichoderma sp can tolerate lower aw. Water activity should be regarded as an external parameter like pH or temperature influencing microbes behaviour in a defined environment. Fungal growth and activity will depend on the water content of the medium but essentially on the form as the water present is (free water or bind water). Pires et al., 2007 observed that during cork slabs resting period after boiling, about 40h were necessary to attain 0.9 aw, in the case of the cork outer bark while in inner bark that value was attained earlier, about 20h after boiling. The authors suggested that fungal spores inside cork can start to germinate before the mycelium appearance on the cork slabs, once 0.9 aw inside cork is attained sooner, and they only will appear on the outer bark after C. sitophila inhibition when lower aw values are attained. As a result of these experiments they have proposed that slabs resting stage should be reduced to two or three days, maximum, before the next stages of cork stoppers manufacturing, in order to reduce fungi development and the possibilities of the associated TCA occurrence. In fact, below 0.9 aw, several fungi genera, especially Penicillium, coexist with C. sitophila (Danesh et al., 1997; Oliveira et al., 2003).
The presence of fungal growth inside cork cells was observed using scanning electron and fluorescence microscopy (Silva Pereira et al. 2006). The authors have observed that fungal growth occurred primarily on the surface of the cork pieces, but mycelium extended deeper into the cork layers, mainly via the cork lenticular channels and also by hyphal penetration of the cork cell wall. The same authors have also reported some results regarding the impact of cork fungal colonisation on the mechanical-physical properties of cork concluding that all colonizing fungi but C. sitophila, reduced cork strength, markedly altering its viscoelastic behaviour and reducing its elasticity (Young's modulus). Furthermore, additional studies have to be performed aiming to better understand fungal colonising correlation to cork mechanical performance. Also deeper studies aiming to better understand the interactions among the different fungi inside the particular niche that cork represents are necessary. These studies are complex as fungal behaviour is normally dependent on either medium composition or culture conditions (e.g. pH, wa, temperature, light). Secondary metabolites like extrolites, VOCs or mycotoxins produced by each member of the cork fungal community can act on the neighbouring members (favouring or inhibiting the respective growth and or metabolism) so acting as cell signalling factors between the community members and as a consequence on all the cork chemical and mechanical properties. Fig. 2 represents an example of fungal interactions, in a semi synthetic culture medium (Potato Dextrose Agar - PDA) (Figure 2A) and in a cork based medium (Figure 2B) clearly showing the culture medium conditions on fungal behaviour.
Cork is a really sustainable product and cork harvesting is an environmentally friendly process during which not a single tree is cut down. The cork oak tree is unique because its bark can be stripped every nine years and bark is restored being ready for the next harvesting about each nine years; an average tree, which lives for 170 to 200 years and can be harvested about 17 times, producing around 4,000 corks per harvest. Moreover, cork oak forest ('montado') contributes for continuous sustainable cork producing, acorn, and wood, fire-wood and also supports the production of mushrooms, honey as well as provides an adequate support to natural pasture. Because of the economic value to local communities, people care for the forests helping to maintain their environmental values as well as reducing the risk of fires and desertification. Additionally, cork oak forests have a critical role to play in the fight against global warming as they are the largest storehouse of carbon on earth. Various studies carried out in Portugal have shown the carbon assimilating and retention capacity of these systems showing that near 1,5 hectares of 'montado' with a tree coverage of, at least, 30 to 40%, are enough to compensate for the annual carbon dioxide emissions of an average vehicle and 118,500 tons of carbon is fixated annually by 15 billion natural cork closures (http://www.realcork.org/artigo.php?art=454).
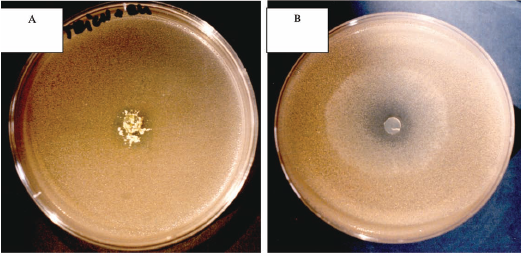
Fig. 1 - A.- Trichoderma longibrachiatum inhibition by Rhodatorula glutinis; B - Mucor plumbeus inhibition by Rhodatorula glutinis
A. Inibição de Trichoderma longibrachiatum por Rhodatorula glutinis; B Inibição de Mucor plumbeus por Rhodatorula glutinis
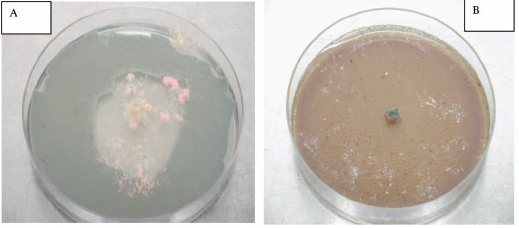
Fig. 2 - A - Trichoderma sp growth inhibition by Chrysonilia sitophila in PDA, 72h, 25ºC. B - Chrysonilia sitophila growth inhibition byTrichoderma sp in a cork based medium, 72h, 25ºC.
A - Inibição do crescimento de Trichoderma sp por Chrysonilia sitophila em PDA. B - Inibição do crescimento de Chrysonilia sitophila por Trichoderma sp num meio contendo cortiça em pó como único substrato.
The noble product from cork oak bark is the cork stopper used for sealing wines due to the chemical and structural composition of the cork oak cells that are responsible for cork unique mechanical properties as referred above. Cork for bottle stoppers accounts for almost 70% of the total value of the cork market. Over 15 billion cork stoppers are produced every year and sold worldwide to the wine industry. Moreover, cork low thermal conductivity, high acoustic and shock isolation capabilities allow its use either in construction or car industry.
The increase in the market of alternative wine stoppers can reduce the economic value of cork forest therefore leading to their degradation, and finally loss of one of the best and most valuable examples of a human-nature balanced system. Consequently, studies focused in the improving of cork stoppers manufacturing, that should be implemented by industry investigation in cooperation with research institutions, are essential to better understand the role of cork fungal colonizer community in the cork stoppers properties. Although the economic sustainability of cork oak depends greatly upon the price of cork, the profitability of different methods of controlling and protecting oak trees depend also on the way as society valuates the supplies and services provided by cork oak forest (http://www.decon.uevora.pt/get_file.php3?id=1185).
Conclusions
At the present moment of Earth civilisation/ sustainability a complete change in the lifestyle at different levels is essential to preserve the planet life. The biodiversity preservation either of animals, plants or microorganisms and the use of alternative ways to explore their respective potential richness in a balanced manner implies deeper research in these areas covering the different required subjects. Therefore, any effort of the international scientific community in this approach is vital to guarantee the global well being.
Micro organisms are important sources of knowledge being of critical importance to the sustainability of life on Earth. They can develop the basic types of metabolism and a wide range of activities, which allow them to colonize all ecosystems. The associations of microorganisms with hosts and other habitats are critical determinants for many topics relevant for mankind such as the quality of the environment, global change, or the decline of food. Within microbial communities different strategies can be developed by the community members in order to ensure their survival. New tools are being applied to overcome the lack of knowledge in this area aiming to enable microbial ecologists to understand and explain the interactions between and among microorganisms and between microorganisms and their physical environment.
The studies summarized on this paper respecting to cork microbial colonisation, tried to illustrate the relationship between microbes, effects on ecosystems behaviour, technological implications on human activities and subsequent economic impact. Besides the cork oak forests critical role to play in the fight against global warming as they are the largest storehouse of carbon on earth, fungal cork slabs colonisation has acquired increased importance as fungi are considered the major responsible by cork (and subsequent wine) spoil, leading to high economic losses involving both cork stoppers and wine industries. This fact had a high negative impact on the use of cork as the preferred wine stopper because since the non producing cork countries have started to develop synthetic materials to replace the traditional cork stoppers. Besides the interference of his kind of sealing devices on the wine quality it is necessary to stress some aspects that may be taken into account when choosing the material to be used: i) cork is a completely biodegradable material, so cork is the only bottle stopper with minimum-impact on the environment while plastic stopper or aluminium screw caps, although recyclable, both need a lot of energy for their production, with the corresponding levels of greenhouse gas emission while cork trees, in contrast, provide a major benefit in absorbing millions of tons of carbon dioxide from the atmosphere. Moreover, the increase in the market of alternative wine stoppers can reduce the economic value of cork forest therefore leading to their degradation, and finally loss of one of the best and most valuable examples of a human-nature balanced system.
Although this is not the scope of this work, it should be stressed here the requirement of increased research efforts aiming to better understand the true role of cork fungal colonisation and to define suitable strategies to preserve cork stoppers use to seal wine bottles.
The concluding remarks to the subject approached along this short review are, once again, well defined in the several texts available from the web site of Convention on Biological Diversity. Below it is presented a copy of one of those texts: 'Although still in its infancy, the Convention on Biological Diversity is already making itself felt. The philosophy of sustainable development, the ecosystem approach, and the emphasis on building partnerships are all helping to shape global action on biodiversity. The data and reports that governments are gathering and sharing with each other are providing a sound basis for understanding the challenges and collaborating on the solutions.
Much, much more needs to be done. The passage of the Earth's biodiversity through the coming century will be its most severe test. With human population expected to rise dramatically, particularly in developing countries, and the consumer revolution set for exponential expansion - not to mention the worsening stresses of climate change, ozone depletion, and hazardous chemicals - species and ecosystems will face ever more serious threats. Unless we take action now, children born today will live in an impoverished world.
The Convention offers a comprehensive, global strategy for preventing such a tragedy. A richer future is possible. If governments and all sectors of society apply the concepts embodied in the Convention and make the conservation and sustainable use of biological diversity a real priority, we can ensure a new and sustainable relationship between humanity and the natural world for the generations to come'.
It is never enough to stress the vital importance of put into practice the approved decisions signed by different countries either at governmental or local level.
REFERENCES
Allen M. R., Lord R. 2004. The blame game - Who will pay for the damaging consequences of climate change? Nature, 432, 551552
Alvarez-Rodriguez M.L., Lopez-Ocana L., Lopez-Coronado J.M., Rodriguez E., Martinez M.J., Larriba G., Coque J.-J.R. 2002. Cork taint of wines: role of the filamentous fungi isolated from cork in the formation of 2,4,6-trichloroanisole by o-methylation of 2,4,6trichlorophenol. Applied and Environmental Microbiology, 68, 5860-5869.
Austin M. 2007. Review-Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecological Modelling, 200, 1-19
Bertin P. N., Médigue C., Normand P. 2008. Advances in environmental genomics: towards an integrated view of microorganisms and ecosystems. Microbiology, 154, 347-359
Brown S. P., Inglis R. F, Taddei F. 2009. Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evolutionary Applications, 2, 1, 32-39
Carney K. M., Matson P. A. 2005. Plant Communities, Soil Microorganisms, and Soil Carbon Cycling: Does Altering the World Belowground Matter to Ecosystem Functioning? Ecosystems, 8: 928-940
Colwell R R 1997. Microbial diversity: the importance of exploration and Conservation. Journal of Industrial Microbiology & Biotechnology, 18, 302-307
Cooper G. 1993.The Competition Controversy in Community Ecology. Biology and Philosophy, 8, 359-384,
Coque J.-J.R., Alvarez-Rodriguez M.L., Larriba G. 2003. Characterization of an inducible chlorophenol O-methyltransferase from Trichoderma longibrachiatum involved in the formation of chloroanisoles and determination of its role in cork taint of wines. Applied and Environmental Microbiology, 69, 5089-5095
Curtis T. P., Sloan W.T. 2005. Exploring Microbial Diversity- A Vast Below. Science, 309, 26, 1331-1333
Danesh, P., Caldas, F. M. V., Figueiredo Marques, J. J., San RomãoM.V. 1997. Mycobiota in Portuguese "normal" and "green" cork throughout the manufacturing process of stoppers. Journal of Apllied Microbiology, 82: 689 - 694.
Fraser C., Alm E.J.., Polz M.F., Spratt B. G., Hanage W. P. . 2009. The Bacterial Species Challenge:Making Sense of Genetic and Ecological Diversity. Science, 323,741-746
Gans J., Wolinsky M,, Dunbar J. 2005. Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil. Science 309, 1387 -1390.
Gaki A., Theodorou A., Vayenas D. V., Pavlou S. 2009. Complex dynamics of microbial competition in the gradostat. Journal of Biotechnology, 139, 38-46
Goldman R. P., Brown S. P. 2009. Making sense of microbial consortia using ecology and evolution. Trends in Biotechnology, 27, 1, 3-4
Hamdallah Z. http://www.wfcc.info/ICCC7/keylectu.html
Harmand J., Rapaport A., Dochain D., Lobry C. 2008. Microbial ecology and bioprocess control: Opportunities and challenges. Journal of Process Control, 18, 865-875
Heilmann-Clausen J., Boddy L. 2005. Inhibition and stimulation effects in communities of wood decay fungi: exudates from colonized wood influence growth by other species. Microbial Ecology, 49, 399-406
Höller U., Wright A. D., Matthée G. F., Konig G. M., Draeger S., Aust H-J., Schulz B. 2000. Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycological Research, 104, 11, 1354-1365
Kennedy J., Marchesi J. R., Dobson A. D. W. 2007. Metagenomic approaches to exploit the biotechnological potential of the microbial consortia of marine sponges. Applied Microbiological Biotechnology, 75, 11-20
Konopa A., 2006. Microbial ecology:searching for principles. Microbe, 1, 4,175-179
Kursar T. A. Caballero-George C. C., Capson T. L, Cubilla-Rios L., Gerwick W. H. , Gupta M. P., Ibañez A. , Linington R. G., Mcphail K. L., Ortega-Barría E., Romero L. I., Solis P. N., Coley
P. D. 2006. Securing economic benefits and promoting conservation through bioprospecting. BioScience, 56, 12, 10051012
Lee T. H., Simpson R. F. 1993. Microbiology and chemistry of cork taints in wine. In Wine Microbiology and Biotechnology. Ed. Fleet, G. H., 353 - 372. Chur, Harwood Academic Publishers
Lefebvre A., Riboulet J. -M., Boidron J.-N.,Ribéreau-Gayon, P. 1983. Incidence des micro-organismes du liège sur les altérations olfactives du vin. Science des Aliments, 3, 265 - 278
Lobry C., Harmand J. 2006. A new hypothesis to explain the coexistence of n species in the presence of a single resource. Comptes Rendus Biologies, 32, 9, 40-46
Maherali H., Klironomos J.N. 2007. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science, 316, 1746-1748.
Masson-Boivin C., Giraud E., Perret X., Batut, J. 2009. Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends in Microbiology, 17, 10, 458-466.
Mazmanian S.K., Round J.L., Kasper D. L. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature, 453, 620-625
McNeal K. S., Herbert B. E. 2009. Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. Soil Science Society of America Journal. 73, 579-588
Miller T. E., Burns J.H., Munguia P., Walters E.L., Kneitel J. M., Richards P. M, Mouquet N., Buckley H.L. 2005. A critical review of twenty years' use of the resource-ratio theory. The American Naturalist. 165, 4, 439-448
Miyazaki M. 2006. Economic value of microbial resources. Microbiol. Cult. Coll. 22, 1, 15?19
Mohanty G., Mukherji S. 2008. Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia, International Biodeterioration and Biodegradation., 61, 240-250
Noah F., Grandy A. S., Six J., Paule E. A. 2009. Searching for unifying principles in soil ecology. Soil Biology & Biochemistry, 41,11, 2249-2256
Oliveira A. C., Peres C. M., Pires, J. M. C., Vitorino S., Figueiredo Marques, J. J. Barreto Crespo, M. T., San Romão M.,V. 2003. Cork stopper industry: defining appropriate mould colonization. Microbiological Research, 158,117-124
Pereira H. 2007. Cork: Biology, production and uses336 p. Amsterdam, Elsevier.
Pires J. M. C., Pereira H., San Romão M. V.. 2007. Study of humidity and water activity of cork slabs during cork stopper manufacturing process - preliminary results. Ciência Técnica Vitivinícola. 22, 1, 15-20.
[ Links ]Pringle A., Bever J. D., Gardes M., Parrent J. L., Rillig, M. C., Klironomos J. N. 2009. Mycorrhizal symbioses and plant invasions. Annual Review of Ecology, Evolution, and Systematics. 40, 699-715
Prak S., Gunata Z., Guiraud J.-P., Schorr-Galindo S. 2007. Fungal strains isolated from cork stoppers and the formation of 2,4,6trichloroanisole involved in the cork taint of wine. Food Microbiology, 24, 271-280
Prat C.l, Olaya R.-R., Rosalia T., Enriqueta A., Dimitra C., Mark S., Baneras L. 2009. Molecular fingerprinting by PCR-denaturing gradient gel electrophoresis reveals differences in the levels of microbial diversity for musty-earthy tainted corks. Applied and Environmental Microbiology, 75, 7, 1922-1931
Prosser J.I., Bohannan B. J. M., Curtis T.P., Ellis R. J., Firestone M. K., Freckleton R. P., Green J. L., Green L. E., Killham K., Lennon J. J., Osborn A. M, Solan M., van der Gast C. J., Young J. P.W. 2007. The role of ecological theory in microbial ecology. Nature Reviews - Microbiology, 5, 384-392
Quince C., Curtis T. P., Sloan W. T. 2008. The rational exploration of microbial Diversity. The ISME Journal, 2, 997-1006
Raffaelli D. 2004. How Extinction Patterns Affect Ecosystems. Science, 306, 1141-1142.
Ragauskas A. J., Williams C.K., Davison B. H., Britovsek G., Cairney J., Eckert C. A., Frederick Jr., W..J., Hallett, J. P., Leak
D. J., Liotta, C. L., Mielenz J. R., Murphy R., Templer R., Tschaplinski T. 2006.The path forward for biofuels and biomaterials. Science, 311, 484-489
Rook G. A. W., Brunet L. R. 2005. Microbes, immunoregulation, and the gut. Gut. 54, 3, 317-320
Sánchez C. 2009. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances, 27, 185-194
Sapp J. 2007. The structure of microbial evolutionary theory. Stud. Hist. Phil. Biol. & Biomed. Sci. 38, 780-795
Scheiner S. M., Willig M. R. 2008. A general theory of ecology. Theor Ecol, 1, 21-28
Silva Pereira C., Soares G.A. M. , Oliveira A.C., Rosa M.E., Pereira H., Moreno N., San Romao M.V. 2006 Effect of fungal colonization on mechanical performance of cork International Biodeterioration & Biodegradation, 57, 244-250
Silva Pereira C., Pires A., Valle M., Vilas-Boas L., Figueiredo Marques, J. J., San Romão, M.V. 2000. Role of Chrysonilia sitophila on the quality for cork stoppers for sealing wine bottle. Journal of Industrial Microbiology and Biotechnology, 24, 256-261.
Sloan W.T, Woodcock S., Lunn M., Head I.M., Curtis T.P. 2007. Modeling taxa-abundance distributions in microbial communities using environmental sequence data. Microbial Ecology, 53, 443-455.
Stephanopoulos G. 2007. Challenges in Engineering Microbes for Biofuels Production. Science 315, 801-804
Teste F.P., Simard S.W., Durall D. M. 2009. Role of mycorrhizal networks and tree proximity in ectomycorrhizal colonization of planted seedlings. Fungal Ecology, 2, 1, 21-30
Thuiller W. 2007. Biodiversity: Climate change and the ecologist. Nature, 448, 7153 , 550-552
Toljander Y. K., Lindahl B. D., Holmer L, Hogberg N.O. S. 2006. Environmental fluctuations facilitate species co-existence and increase decomposition in communities of wood decay fungi. Oecologia, 148, 625-631
Tscharntke T., Bommarco R., Clough Y.., Crist T. O., Kleijn D., Rand T. A., Tylianakis J. M., van Nouhuys S., Vidal S. 2007. Conservation biological control and enemy diversity on a landscape scale. Biological Control, 43, 3, 294-309
Velicer G. J. 2003. Social strife in the microbial world. Trends in Microbiology, 11, 7, 330-337
Welbaum G. E., Sturz A. V.,Dong Z., Nowak J. 2004. Managing soil microorganisms to improve productivity of agro-ecosystems. Critical Reviews in Plant Sciences, 23, 2, 175-193
West S. A., Griffin A. S., Gardner A., Diggle S. P. 2006. Social evolution theory for microorganisms. Nature Reviews Microbiology, 4, 597-607
Widen P. 1997. Competition and the fungal community, In Environmental and microbial relationships. The Mycota IV. 135-147. Wicklow D. T. and Soderstrom B. (vol. ed.) - Esser K. and Lenke P. A. (ed.) Springer-Verlag, New York, USA).
http://www.cbd.int/convention/convention.shtml
http://www.celiege.com/Ingles/systecode/international_code/international_code.pdf
http://www.realcork.org/artigo.php?art=14













