Introduction and Objectives
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are well-established resection techniques for colorectal superficial tumors, depending on their size, morphology, and localization [1-4]. For larger lesions (>40 mm diameter) and whenever superficial submucosal cancer is suspected or cannot be firmly excluded, ESD is the recommended approach, as it allows en bloc resection regardless of the lesion’s size, leading to a minimized recurrence risk [5, 6].
In regard to low rectal lesions extending to the anorectal junction (ARJ) lesions, ESD could be more challenging. Indeed, in contrast to more proximal colorectal lesions, ARJ lesions could theoretically present a higher risk of bleeding (due to the presence of the rectal venous plexus) and post-procedural pain (given the presence of sensory nerves in the squamous epithelium of the anal canal). Moreover, local direct drainage into the systemic circulation could increase the risk of bacteremia. Additionally to these anatomical features, the narrow lumen and permanent contraction of the anal sphincter could also impair the endoscopic diagnostic accuracy and increase the resection technical difficulty due to poor maneuverability and reduced visualization of the lesion and resection field.
Randomized trials comparing different local resection techniques (EMR, ESD, and transanal surgery) are lacking, and the optimal management strategy in this context still remains to be determined. This multicenter cohort study aimed to evaluate the feasibility, safety, and efficacy of ESD for ARJ neoplastic lesions (<20 mm from the dentate line) in comparison to more proximal rectal lesions (>20 mm from the dentate line).
Material and Methods
Patient Selection and ESD Technique
We included all ESDs performed consecutively in two referral European centers (Gastroenterology Department of Centro Hospitalar Universitário S. João, Porto, Portugal, and Department of Gastroenterology, Hepatopancreatology, and Digestive Oncology, CUB Erasme Hospital, Université Libre de Bruxelles [ULB], Brussels, Belgium), from January 2015 to June 2021. Patients’ data were prospectively recorded in an electronic database and retrospectively reviewed for this study. Written informed consent was ob-tained from every patient before ESD. The institutional Ethics Board of both centers approved the prospective collection and retrospective analysis of the included data.
Lesions selected for ESD resection included neoplastic epithelial rectal lesions (from the anal verge until 15 cm from this site) that had no endoscopic suspicion of deep submucosal invasion and were unsuitable for en bloc EMR. For the purpose of the study, all the lesions totally or partially located within less than 2 cm from the dentate line were considered “ARJ lesions,” as suggested by previous reports [7]. This is where the anal transition zone is most commonly found, with the mucosal folds (Morgagni columns) and the hemorrhoidal plexus usually unfolding distally from there. The remaining lesions were called “rectal lesions.” The procedures were performed using the GIF-H190 gastroscope (Olympus®, Tokyo, Japan). High-definition endoscopy, dye chromoendoscopy, and/or narrow-band imaging were used for characterization of all the lesions. Dissection was performed using 1.5- or 2-mm dual knives (Olympus®, Tokyo, Japan) for mucosal incision. Dual knives, insulated tip-2, or insulated tipnano knives (Olympus®, Tokyo, Japan) were used for submucosal dissection. Erbe ICC-200, ICC-300, VIO-300, or VIO-3 electrosurgical units (ERBE® Elektromedizin GmBH, Tubingen, Germany) were used, with ENDO CUT mode effect 2 or DRY CUT mode effect 2, 30 W for mucosal incision and forced or swift coagulation (effect 3 or 4, 30 W) for submucosal dissection. Hemostasis (soft coagulation effect 5, 50-80 W) was performed with a Coagrasper (Olympus®, Tokyo, Japan), whenever necessary and at the end of each procedure. There was no predefined ESD strategy, and each operator had the liberty to choose the best strategy and approach to each lesion, depending on his/her own experience and the lesion’s presentation. Classic and tunnel strategies were mostly applied. All patients received antibiotic prophylaxis in case of anal canal involvement (either with amoxicillinclavulanic acid [1,000 mg-62.5 mg] or with ceftriaxone [1 G] and metronidazole [500 mg]).
After ESD, all patients were followed in the outpatient clinic (with an appointment approximately 1 month after the procedure). Light analgesics (paracetamol) were prescribed at discharge, to be taken only in case of any pain or discomfort. Patients were also instructed to immediately call the department if any clinical abnormality appeared (including pain, bleeding, fever, or any other discomfort). Endoscopic follow-up was performed 3-6 months after ESD and posteriorly according to the current surveillance guidelines. Patients with malignant lesions were always discussed in a multidisciplinary dedicated board.
Histopathological Evaluation
ESD specimens were sent to pathology evaluation with pins on a cork plate, fixed in formalin. Sectioning at 2-mm intervals was performed to evaluate lateral and vertical margins.
Definitions and Outcomes
ESD failure was determined whenever the target lesion was not removed. En bloc resection required that the target lesion be retrieved in one single specimen, as opposed to a piecemeal resection (if the lesion was removed in more than one fragment). R0 resection was achieved when pathological evaluation showed free horizontal and vertical margins (even if there was <1 mm of normal tissue between the margins and the lesion) in an en bloc resected specimen. Specimens with thermal effects at the margins preventing the pathologist from definitely excluding the presence of abnormal cells were considered R1 resections. The area of the ESD specimen was calculated as the surface of an ellipse, multiplying half of the larger side with half of the smaller side with pi-value and expressed in mm2. ESD velocity was calculated by dividing ESD area by the time of procedure in minutes (mm2/min).
In regard to adverse events, perforation was defined as the visualization of the mesorectum or intra-abdominal cavity during the procedure. Every case of post-procedural bleeding (rectorrhagia) was registered, regardless of the severity. Procedure-related mortality was defined as any death resulting from the ESD procedure.
Statistical Analysis
Categorical variables were described as absolute (n) and relative frequencies (%). Mean and standard deviation or median and percentiles or range were used for continuous variables as appropriate. When testing a hypothesis about continuous variables, t-Student or Mann-Whitney tests were used as appropriate, considering normality assumptions and the number of groups compared. When testing a hypothesis about categorical variables, a χ2 test and Fisher’s exact test were used, as appropriate. The significance level used was 0.05. Statistical analysis was performed using Statistical Package for the Social Sciences v.25.
Results
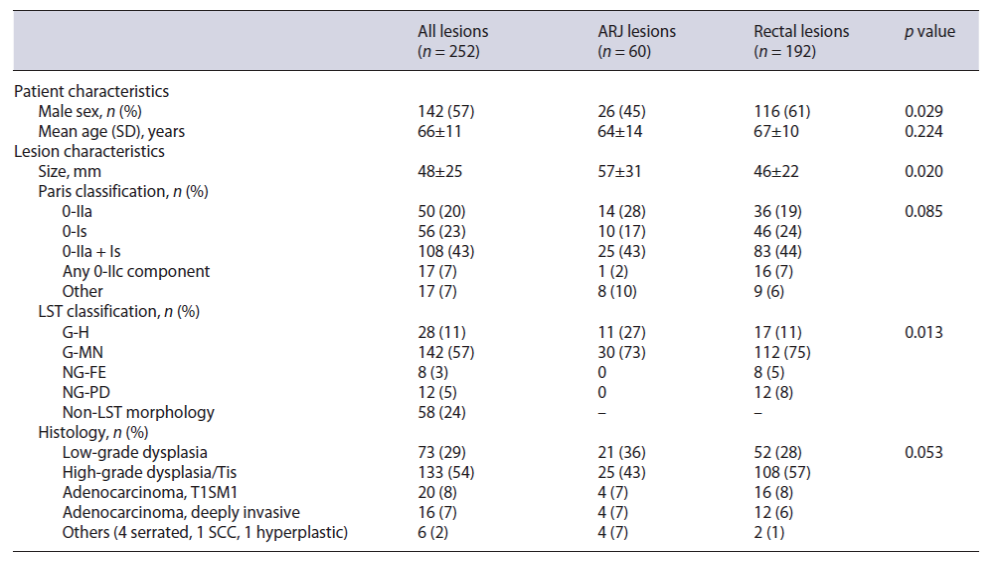
Two hundred and fifty-two lesions were included in the study, with a mean age of 66 ± 11 years old and including 142 (57%) males. Sixty (24%) were located in the ARJ (Fig. 1), and the remaining 192 were located more proximally in the rectum. The mean lesion size was 48 ± 25 mm. Twenty-one (8%) lesions had already a previous resection attempt by EMR. In regard to morphology, the majority (43%) were Paris IIa + Is lesions, and only 10%were non-granular LSTs (Table 1). Technical success was achieved in 248 procedures (98%), 58 in the ARJ, and 190 in the rectum. En bloc resection was achieved in 97% of all cases and R0 resection in 75%. The median procedure time was 90 min (IQR 60-150 min). Hybrid ESD was re-quired in 8 cases (3%), and the pocket-creation method was used in only 3 lesions (1%).

Fig. 1 A case of a granular mixed nodular lateral spreading tumor located at the ARJ. a Lesion extending to the dentate line. b Cut in the anal squamous mucosa. c Mucosal defect in the anal canal. d Final mucosal defect. Final histology shows SM1 submucosal adenocarcinoma.
As opposed to the Belgian practice (where it’s protocol for all patients to stay for overnight in-hospital observation), in the Portuguese center, rectal ESDs are routinely performed in an ambulatory setting, which is why 123 out of 161 (76%) patients were discharged after only 4-6 h observation. The remaining patients (n = 38) were admitted for longer observation due to extensive resection (n = 30), severe intraprocedural bleeding (n = 5), and intra-procedural perforation (n = 3). None of the patients were admitted due to post-procedural anal pain. Taking into consideration the patients from the two centers, most of those requiring hospital admissions (86%) were admitted for strict surveillance during 1 day only.
Regarding histopathology, most of the cases presented high-grade dysplasia/Tis adenocarcinoma (54%). Thirty-six (15%) had submucosal adenocarcinoma, including 20 superficial (sm1) and 16 deeply invasive (>SM1) T1 cancers. According to a multidisciplinary board decision, 13 patients were submitted to surgery due to a non-curative ESD, with 2 patients presenting lymph node metastasis but none with residual intramural dysplasia in the surgical specimen. From the remaining, 165 patients have already been submitted to follow-up endoscopies, with a median follow-up time of 12 months (IQR 6-18.5 months) and including 71% of the ARJ lesions and 64% of the rec-tal lesions group (p = 0.321). In total, only 2 patients presented residual lesions during follow-up.
Adverse Events
A total of 10 intraprocedural perforations (4%) were observed, all of them endoscopically resolved by the application of hemostatic clips. None of the patients required surgery due to an intraprocedural adverse event, and mortality was 0%.
Twenty patients (8%) developed post-procedural bleeding. Of them, only 3 patients needed endoscopic clipping; in the remaining cases, bleeding had stopped at the time of the rectoscopy and no treatment was required. Seven patients (3%) developed post-procedural anal pain, which resolved with systemic or topic analgesics, and 7 (3%) developed fever in the first day after the ESD that resolved with paracetamol.
Comparison between ARJ Lesions and Rectal Lesions
We found no differences between the location of the lesions regarding intraprocedural bleeding, en bloc resection, R0 resection, curative resection, duration of ESD, area of the lesions, ESD velocity, or histopathology result, as well as the need of surgery due to a non-curative ESD (Table 2). Also, there were no statistically significant differences regarding the hospitalization rate, delayed bleeding, perforation, fever, and the need of readmission due to adverse events.
Three patients from the ARJ lesions group developed post-procedural stenosis (5%), against none in the rectal lesions group (p = 0.003). Also, 5 patients with ARJ lesions (9%) reported anal pain, in opposition to only 2 (1%) from the rectal lesions group (p = 0.002).
From those followed up by endoscopy, 2 patients had residual lesion (1 in the first endoscopy following the ESD procedure at 15 months and 1 in the second follow-up at 17 months). They were both low-grade dysplastic adenomas and occurred in patients with ARJ lesions (p = 0.064) that had positive horizontal margins in the ESD. From those submitted to surgery (n = 13), the only 2 patients with lymph node metastasis belong to the rectal lesions group (p = 1.000). At ESD pathological evaluation, one had positive lateral and vertical margins and tumor budding, while the other had deep submucosal invasion and budding.
Discussion and Conclusions
In our study, we performed a comparative evaluation of 252 rectal lesions treated by ESD, including 60 ARJ lesions and 192 non-ARJ lesions. We found similar results in regard to en bloc resection, R0 resection, and curative resection rates in both groups, independent of the rectal location. Similarly, the rates of residual lesion and overall complication rates were comparable between the two groups, except for a higher rate of post-procedural stenosis (p = 0.003) and anal pain (p = 0.002) in the ARJ lesions group. As reported in the literature [8], most of the patients with ARJ lesions were female, contrary to those located elsewhere in the rectum.
Regarding efficacy, Imai et al. and Probst et al. [8, 9] had previously consistently described a lower rate of R0 resections in ARJ lesions, probably due to thermal damage at the anal side of the resection specimen. In our co-hort, we did not verify this, as R0 resection rate was similar in both groups of patients, possibly reflecting an increased operator experience and improvements in pathology evaluation.
Recently, EMR effectiveness for the treatment of ARJ lesions has been reported [7, 10]. Nevertheless, in ARJ location, ESD shows the same advantages over EMR that had already been established for other colorectal regions [11, 12]. Mainly, it allows for an en bloc resection regardless of the lesion’s size, resulting in a more accurate staging for invasive lesions. This is especially important in ARJ lesions, given the increased difficulty in making an accurate and qualitative endoscopic diagnosis of the lesions in this area, as a consequence of the narrow and constrictive character of the anal canal. Also, complementary surgical procedures after a non-curative ESD could harbor much more morbidity in the case of ARJ lesions comparing to other locations in the rectum, which highlights the importance of a R0 resection. Furthermore, ESD implies a direct vision cut and thus enables for a precise dissection above the vascular plexus, besides allowing the rigorous definition of the resection line and preventing the resection of excessive surrounding healthy tissue, thus minimizing mucosal defects and potentially reducing the risk of post-procedural stricture.
The number of patients that have been submitted to complementary surgery or endoscopic follow-up and demonstrated residual lesions was very low, as previously reported [13]. The only 2 cases of residual lesion corresponded to ARJ benign lesions, but they were both non-curative resections from the start. Therefore, we did not find any difference between the two locations, as supported by previous studies [8].
The risk of procedure-associated complications like bleeding, infection, pain, and stenosis has been of major concern. In our series, patients with ARJ lesions showed a higher incidence of post-procedural pain (p = 0.002), which is explained by the presence of nociceptive receptors in squamous epithelium on the distal margin. Previous authors have proposed that the injection of lidocaine to the submucosal layer could reduce post-procedural pain, even though strong data supporting this strategy are still lacking [14, 15]. Stenosis incidence was also significantly higher in the ARJ group (p = 0.003), which could be associated to the significantly greater diameter of the lesions included in this group comparing to more proximal rectal lesions (57 ± 31 mm vs. 46 ± 22 mm, p = 0.02) and to the smaller size of the luminal circumference in the distal rectum and canal anal. In regard to post-procedural bleeding and infection, there were no significant differences between groups, emphasizing the safety of ESD for ARJ lesion.
Our study is currently the largest series addressing ESDs in the ARJ. However, it has some limitations, including the retrospective design, even if based upon prospectively collected data, and the short follow-up period, which could lead to delayed complications rate and recurrence underestimation. Also, all procedures were performed by experienced endoscopists only, not allowing extrapolation for less experienced operators.
In conclusion, ESD is a safe and efficient technique for the treatment of rectal lesions located in the ARJ, similarly to those located elsewhere in the rectum. Randomized controlled trials comparing ESD and EMR are warranted to address the best approach for these lesions.