Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. vol.27 no.2 Dois Portos dez. 2012
Evaluation of fermenting grape must yeast dynamics by SSCP profiles
Avaliação da dinâmica de leveduras presentes na fermentação de mostos por perfis de SSCP
Filomena L. Duarte*, Margarida Baleiras-Couto
Instituto Nacional de Investigação Agrária e Veterinária, I.P (INIAV, I.P.). Quinta da Almoinha, 2565-191 Dois Portos. Portugal
*Corresponding author:
SUMMARY
Alcoholic fermentation of grape must is a dynamic process involving diverse yeast populations which play an important role in wine characteristics. In order to enable a permanent control by the oenologist rapid and low cost methodologies to identify the species of yeasts present during fermentation should be implemented. Single Strand Conformation Polymorphism technique (SSCP), targeting D1 and D2 domains of 26S rDNA, was tested to differentiate wine yeast species. SSCP profiles were produced for 17 collection strains belonging to 15 different wine related yeast species. The technique was further investigated for the identification of yeasts inoculated to sterile grape must as single or in mixtures of two different species. Identical SSCP profiles were obtained from yeasts grown in grape must and in conventional growth media. SSCP allowed obtaining species specific bands, and SSPC profiles from grape must inoculated with mixtures of two strains revealed the presence of specific bands of both species. Direct SSCP yeast analysis of a spontaneous fermenting must carried out after 48 and 192 h of fermentation, enabled to compare the yeast community at two different periods of fermentation. SSCP profiles showed the presence of bands from species associated with the beginning and the end of fermentation, respectively at 48 and 192 h of fermentation. We conclude that SSCP is a quite promising methodology to monitor yeast populations present during grape must fermentation, which revealed to be easy to implement and low-priced.
Key words: SSCP, rDNA, yeast, population dynamics, grape must fermentation.
RESUMO
A fermentação alcoólica do mosto de uva é um processo dinâmico que envolve diversas populações de leveduras que desempenham um papel importante nas características do vinho. A fim de permitir um controle permanente pelo enólogo urge implementar metodologias rápidas e de baixo custo, para identificação das espécies de leveduras envolvidas na fermentação. O método de Polimorfismo de Conformação do DNA de Cadeia Simples (SSCP) visando os domínios D1 e D2 da região 26S do rDNA foi testado para diferenciar espécies de levedura associadas ao vinho. Foram determinados perfis de SSCP para 17 estirpes de coleção pertencentes a 15 espécies diferentes associadas a ambientes vínicos. A técnica foi depois testada para a identificação de leveduras inoculadas em mosto estéril utilizando uma única espécie ou em misturas de duas espécies diferentes. Foram obtidos perfis idênticos de SSCP a partir de leveduras cultivadas em mosto de uva e em meios de cultura convencionais. A análise de SSCP permitiu a obtenção de bandas específicas de cada espécie, e os perfis de SSPC obtidos a partir de mosto de uva inoculado com misturas de duas estirpes de espécies diferentes revelou a presença de bandas específicas de ambas as espécies. A análise direta por SSCP das leveduras presentes numa fermentação espontânea, realizada após 48 horas e 192 h de fermentação, permitiu comparar a comunidade de leveduras em dois períodos diferentes de fermentação. Os perfis de SSCP revelaram a presença de bandas de espécies associadas com o início e com o fim da fermentação, respetivamente às 48 e às 192 h de fermentação. Em conclusão, a análise SSCP é uma metodologia bastante promissora para monitorizar as populações de leveduras presentes durante a fermentação, que se mostrou ser de fácil aplicação e de baixo custo.
Palavras-chave: SSCP, rDNA, leveduras, dinâmica populacional, fermentação de mosto.
INTRODUCTION
Yeasts have a primordial role in wine quality. The presence of different populations of yeast species during grape must fermentation, results in the production of different compounds or different compound proportions, which determines the sensorial characteristics of the produced wine (Ciani and Maccareli; 1998; Jolly et al., 2006; Ciani and Comitini, 2011). The knowledge of which yeast species are involved, as well as their dynamics is thus a key factor to control wine quality.
Molecular biology based methods have been described for the rapid identification of yeast species. Most of these methods such as RAPD, PCR fingerprinting and restriction enzyme analysis of rDNA fragments rely on PCR amplification (Baleiras Couto et al., 1995; 1996; 2005). Nevertheless, these methods require previous isolation and cultivation of single cultures which is very laborious and time consuming. When diversity and dynamics of microbial communities are to be studied, culture independent methods are recommended (Calon et al., 2006; Mounier et al., 2009). Methods such as Single Strand Conformation Polymorphism (SSCP), Real Time PCR (RT-PCR), Denaturing Gradient Gel Electrophoresis (DGGE) or Fluorescence in situ hybridization have been developed in order to allow species identification directly from natural substrates (Cocolin et al., 2000; 2002; Peters et al., 2000; Prakitchaiwattana et al., 2004; di Maro et al., 2007; Renouf et al., 2007; Andorrà et al. 2008; 2012; Mounier et al., 2009).
Compared to other methods SSCP revealed to be less expensive, does not involve complex equipment nor limits the number of species to be identified. SSCP also enables further analysis of individual bands which can be excised for identification by sequencing or reverse transcription PCR followed by sequencing when SSCP is performed over RNA (MacGregor and Amann, 2006). SSCP technique has shown high discriminative power in diverse types of studies, enabling the recognition of medically important opportunistic fungi (Walsh et al., 1995) the profiling of microbial communities from estuarine sediment and hypersaline microbial mats (MacGregor and Amann, 2006) and in the differentiation of representative groups of yeast species from ascomycetes and basidiomycetes (Wang et al., 2008). Yeasts diversity and population dynamics in fermentation of diverse food products has also been evaluated by SSCP analysis (Chamkha et al., 2008; Chang et al., 2008; Mounier et al., 2009). Due to the adequacy of ribosomal DNA to yeasts phylogenetic inferences, the above mentioned works rely on fragments from rRNA gene or rRNA to perform SSCP analysis. The sequence of D1/D2 domains of 26S rDNA has been used for yeast identification (Kurtzman and Robnett, 1998). Previous works on the identification of wine yeasts from different environments have shown that sequencing of these domains or restriction analysis of fragments comprising these domains was very accurate (Baleiras-Couto et al., 2005; Zanol et al., 2010; Baleiras-Couto et al., 2012).
The aim of this work was to investigate the use of SSCP analysis to differentiate wine yeast species and to evaluate the adequacy of the method to fermenting must in order to establish a rapid and inexpensive tool to survey yeast population dynamics during fermentation. SSCP analysis of 26S rDNA amplified fragments (D1 and D2 domains) was performed to characterise 17 different collection yeast strains belonging to 15 wine associated species. SSCP analysis was also carried out directly to grape must inoculated with cultures of one or two yeast species. Additionally the applicability of SSCP analysis was tested in grape must after 48 and 192 h of spontaneous fermentation in order to compare the yeast community present at two different periods of fermentation.
MATERIAL AND METHODS
Yeast strains
The yeast strains used in this study are included in Coleção de Microrganismos EVN (EVN) and were obtained from other culture collections, representing 15 wine related species, as indicated in Table I.
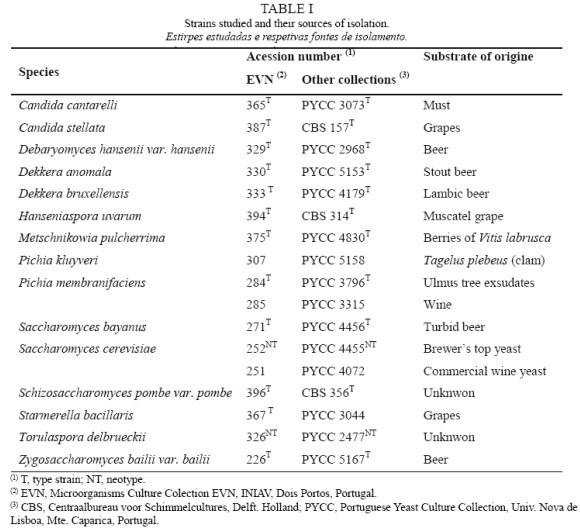
Cells culture
To determine the SSCP profiles of each species in study, yeast cells were grown in conventional agar medium (YPD agar - yeast extract, peptone, dextrose, agar) at 25 ºC.
For cells growth in grape must a loop full of cells was added to test-tubes with 10 ml sterile grape must and incubated at 25 ºC during 48 h without shaking. When cell numbers reached 5 x 106 per ml SSCP profiles of single or mixtures of equal volumes of two cultures were determined.
DNA extraction and PCR amplification
Cells grown on YPD agar were disrupted according to Sampaio et al. (2001). DNA was further purified with chlorophorm: isoamyl alcohol (24:1), precipitated with 1/10 volume of sodium acetate (3 M, pH 5.2) and two volumes of absolute ethanol. Further incubation with RNase (100 µg/mL in TE: 10 mM tris-HCl and 1 mM EDTA, pH 8) was performed for 30 min, at 37 ºC and additional purification and precipitation steps were carried out as described above. DNA was washed with ethanol (70 %, v/v), dried and ressuspended in TE.
For DNA extraction from cells grown on grape must, 2 ml of must with approximately 5 x 106 cel/ml was centrifuged at 18.200g at 4 ºC during 10 min. The pellet was washed with NaCl solution (9 g/L) and ressuspended in 500 µl lyses buffer (Tris 1 M; EDTA 0,25 M; NaCl 5 M; CTAB 0,5 mM; pH 7,5) added with 120 µl of 5% (w/v) N-lauroylsarcosine sodium solution. Then 200 µl glass beads (425-600 µm Ø) were added, vortexed at maximum speed for 1 min and left for 15 min at 65ºC. DNA was purified with chlorophorm:isoamyl alcohol (24:1), precipitated with isopropanol and washed with ethanol (70 %, v/v). Incubation with RNase was also performed and the DNA was precipitated with sodium acetate and isopropanol at -20 ºC overnight. DNA was washed with ethanol (70 %, v/v), dried and ressuspended in TE.
For the amplification of D1/D2 domains of 26S rDNA, PCR conditions were similar to those described by Zanol et al. (2010) using 1 ng of total DNA and primers NL1 (5-GCATATCAATAAGCGGAGGAAAAG-3) and NL4 (5-GGTCCGTGTTTCAAGACGG-3).
SSCP analysis
To denature the amplified fragment, 4 µl of PCR product were mixed with an equal volume of denaturing formamide solution (containing 1 mg/ml xylene cyanol, 1 mg/ml bromophenol blue, 20 µl/ml EDTA 0,5 M). The reaction mixture was denatured by heating at 95 ºC for 5 min in a thermocycler (T Gradient 96 cycler, Whatman-Biometra, Gottingen, Germany) and immediately cooled on ice directly before use. Eight µl of the sample were loaded into each well of a non-denaturing 5 % acrylamide:bis (30:1) gel containing 5 % (v/v) glycerol casted on a Mighty Small II apparatus from Hoefer/Pharmacia (8 x 7 cm and 0.75 mm thick). DNA migration occurs from denaturing (loading solution) to non-denaturing (polyacrylamide gel) conditions. A standard DNA non-denatured marker (1 kb DNA Ladder, MBI Fermentas) was loaded on the first and the last wells of the gel, and used as a reference to determine the relative positioning of the SSCP bands, as well as the double stranded DNA band.
Electrophoresis was performed in 1x TBE (Tris-borate-EDTA) buffer, run at 150 V, at 4 ºC until migration of xylene cyanol dye reached 0.5 cm of the bottom of the gel. The gels were silver stained and photographed. The relative position of SSCP bands, was determined using GelCompar II software, version 5.1 (Applied Maths, Saint-Martens-Latem, Belgium). Gels were repeated at least three times and the bands present on all the gels were attributed to the species.
RESULTS AND DISCUSSION
Analyses of single-strand conformation polymorphism (SSCP) enable DNA fragments with similar size but different sequence to be separated according to their configuration (secondary structure) (Hebenbrock et al., 1995). Several regions of the rRNA gene have proved adequate to discriminate yeast species, namely the D1/D2 domains of 26S rDNA, which was selected to be used in the present work (Kurtzman and Robnett, 1998; Esteve-Zarzoso et al., 1999; Zanol et al., 2010).
In order to initiate a wine yeast species SSCP profile database, 17 culture collection strains belonging to 15 yeast species associated with wine environments were studied. A PCR amplified fragment consisting of the D1 and D2 domains of 26S rDNA was used to perform SSCP analyses. After optimization of procedures, SSCP profiles of each strain grown in YPD agar were generated. Figure 1 shows some of the profiles obtained.
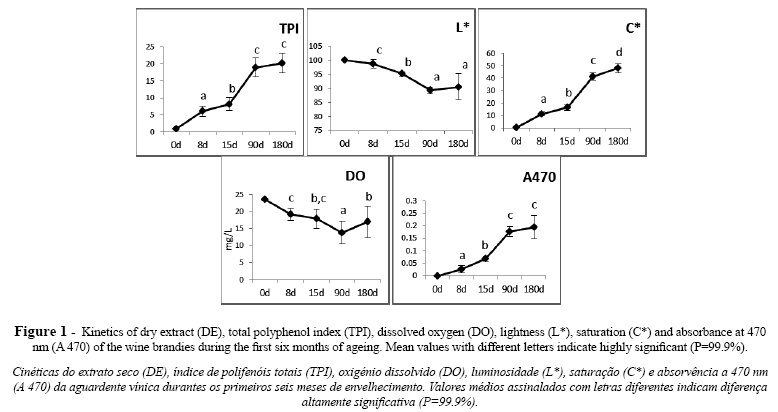
Gels were repeated at least three times from independent cultures. All gels were analyzed and the bands relative positioning determined. Table II shows the values of the SSCP specific bands attributed to each species.
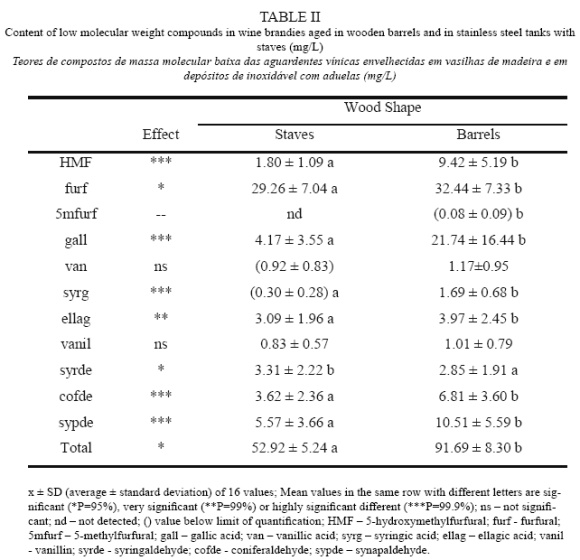
Every profile presented three bands, two of them corresponding to forward and reverse single strand DNA (ssDNA) (Callon et al., 2006). The third band corresponded to double strand DNA (dsDNA) as was verified by electrophoresis of denaturated and non-denaturated amplified DNA (Figure 2). Although this third band is not ssDNA, it appears in SSCP gels (Wang et al., 2008). This band was considered in the profiles since it presented species polymorphism, improving the discriminative power of this methodology.
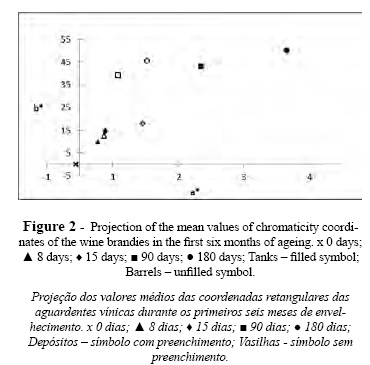
Results showed that the SSPC profiles obtained for different strains of the same species, as was tested for P. membranifaciens and S. cerevisiae, were identical (Table II). A distinct SSCP profile was obtained for the great majority of the species evaluated. Nevertheless, C. stellata and Starmerella bacillaris presented similar profiles. These two species were for long considered to be conspecific and only recently were clearly differentiated by the sequencing of D1/D2 domains, presenting only 91% similarity (Duarte et al., 2012). From an oenological point of view this SSCP profile similarity is not a real problem as it has been shown that C. stellata is rarely found in wine fermentation unlike Starmerella bacillaris which is quite common in wine associated environments (Csoma and Sipiczki, 2008; Duarte et al., 2012). Similar SSCP profiles were produced by the strains of D. anomala and D. bruxelensis, although the sequence of D1/D2 domains of 26S rDNA of these two strains presents a similarity of only 93% (Kurtzman and Robnett, 1998). Nevertheless they could be clearly differentiated from the remaining species. Equivalent results were obtained using restriction profile analysis of 26S rDNA (Zanol et al., 2010). The SSCP showed to be quite discriminative for 11 out of 15 species, which can be clearly differentiated from each other and from the species belonging to the Dekkera genus and the Starmerella clade, C. stellata and Starmerella bacillaris (Lachance et al., 2011).
The applicability of the methodology was also tested upon yeast growth on grape must. Another DNA extraction procedure had to be optimized as no PCR amplification was obtained with the previous one, due to the presence of PCR inhibiting compounds. After optimizing the DNA extraction methodology as described in Material and Methods, SSCP analysis was performed without further modification. The profiles obtained were identical to those obtained after growth in conventional medium (Figure 3).
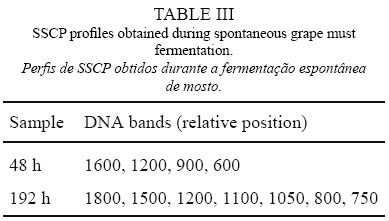
SSCP analysis of mixtures of two yeast strains independently grown in grape must was also performed and the results analyzed using GelCompar II software. The SSCP profiles produced revealed that the assayed mixtures presented a combination of bands corresponding to the specific bands of both species. In Figure 4, SSCP profiles from single and mixed cultures of C. cantarelliplus Starmerella bacillaris and C. stellata plus D. anomala are presented, and bands from both species can be visualized. The extra bands present might be due to the use of rDNA fragments as markers (Wang et al., 2008).In order to test the applicability of this methodology to real conditions, SSCP analyses were also performed using DNA directly extracted from grape must after 48 and 192 hours of spontaneous fermentation. The SSCP profiles showed in Figure 5 were compared with those presented by the culture collection strains. The relative position of the detected bands is also presented at Table III. The SSCP profile of the sample collected after 48 h of fermentation showed bands associated to C. stellata/Starmerella bacillaris and to H. uvarum. After 192 h of fermentation the SSCP analysis generated 7 bands (Table III) which matched the specific bands of S. cerevisiae, S. bayanus, T. delbrueckii, Deb. hansenii and Schizo pombe species. From these results, it can be inferred that grape must at the beginning of fermentation was dominated by Starmerella clade and Hanseniaspora yeasts as observed by others (Fleet, 2003; Baleiras-Couto et al., 2005). Grape must sampling after 192 h pointed out to the presence of bands corresponding to species associated with late grape must fermentations (Fleet, 2003; Baleiras Couto et al., 2005).
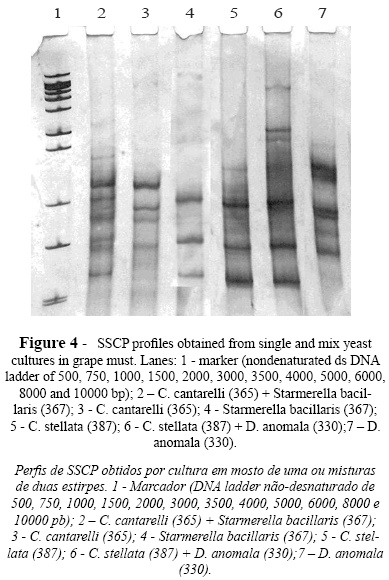
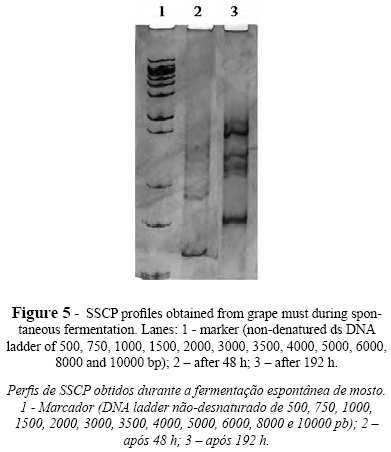
In view of the results obtained, the performance of SSCP to detect wine related yeast species in grape must fermentation was appropriate, leading to its future applicability to study the diversity and dynamics of yeast communities. Likewise, other works using SSCP have been done featuring the study of microbial diversity and population dynamics in various food products such as olive fermentation brine (Chamkha et al., 2008), commercial cheeses (Mounier et al., 2009), or a Korean food fermented from vegetables (Chang et al., 2008). This methodology presents the advantages of overcoming the amount of work associated with the identification of several isolates and the potential biases inherent in microbial enrichment and of detecting viable non-culturable microorganisms (Wang et al., 2008). The SSCP can also be used to evaluate the implantation of inoculated non-Saccharomyces yeasts which is a promising technology yet at the beginning (Ciani and Comitini, 2011).
CONCLUSIONS
SSCP analysis targeting D1/D2 domains of 26S rDNA proved very discriminative for the yeast species in study. The applicability of this methodology directly to fermenting grape must samples was quite good, revealing similar band definition, thus being appropriate for studying the diversity and dynamics of wine yeasts during fermentation. To our knowledge this is the first time that SSCP was used to compare yeast community during grape must fermentation. Future work is envisaged to enlarge the SSCP profile database that expedites the identification process. This technique presents the advantage of obtaining fast results with less expensive equipment required.
ACKNOWLEDGMENTS
The authors would like to thank Joana Duarte and Maria Filomena Alemão for technical assistance.
REFERENCES
Andorrà I., Berradre M., Mas A., Esteve-Zarzoso B., Guillamón J.M., 2012. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT - Food Science Technol., 49, 8-13. [ Links ]
Andorrà I., Landi S., Mas A., Guillamón J.M., Esteve-Zarzoso B., 2008. Effect of oenological pratices on microbial populations using culture-independent techniques. Food Microbiol., 25, 849-856. [ Links ]
Baleiras-Couto M.M., Gomes A.S., Casal M., Duarte F.L., 2012. Survey of yeast diversity during wine bottling processes using restriction analysis of 26S ribosomal DNA (rDNA). Aust. J. Grape Wine Res., 18, 39-42. [ Links ]
Baleiras-Couto M.M., Hartog B.J., Huis in't Veld J.H.J., Hofstra H., van der Vossen J.M.B.M., 1996. Identification of spoilage yeasts in a food-production chain by microsatellite polymerase chain reaction fingerprinting. Food Microbiol., 13, 59-67. [ Links ]
Baleiras Couto M.M., Reizinho R.G., Duarte F.L., 2005. Partial 26S rDNA restriction analysis as a tool to characterise non-Saccharomyces yeasts present during red wine fermentations. Int. J. Food Microbiol., 102, 49-56. [ Links ]
Baleiras-Couto M.M., Vogels J.T.W.E., Hofstra H., Huis int Veld J.H.J. and van der Vossen, J.M.B.M., 1995. Random amplified polymorphic DNA and restriction enzyme analysis of PCR amplified rDNA in taxonomy: two identification techniques for foodborne yeasts. J. Appl. Bacteriol., 79, 525–535. [ Links ]
Callon C., Delbès C., Duthoit F., Montel M.-C., 2006. Application of SSCP–PCR fingerprinting to profile the yeast community in raw milk Salers cheeses. Syst. Applied Microbiol., 29, 172–180. [ Links ]
Chamkha M.; Sayadia S, Brub V., Godon J.J., 2008. Microbial diversity in Tunisian olive fermentation brine as evaluated by small subunit rRNA - Single strand conformation polymorphism analysis. Int. J. Food Microbiol., 122, 211-215. [ Links ]
Chang H.W., Kim K.H., Nam Y.D., Roh S.W., Kim M.S., Jeon C.O., Oh H.M., Bae J.W., 2008. Analysis of yeast and archaeal population dynamics in kimchi using denaturing gradient gel electrophoresis. Int. J. Food Microbiol., 126, 159–166. [ Links ]
Ciani, M., Comitini, F., 2011. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol., 61, 25-32. [ Links ]
Cocolin L., Aggio D., Manzano M., Cantoni C., Comi G., 2002. An application of PCR–DGGE analysis to profile the yeast populations in raw milk. Int. Dairy J., 12, 407–411. [ Links ]
Cocolin L., Bisson L.F., Mills D.A., 2000. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett., 189, 81-87. [ Links ]
Csoma H., Sipiczki M., 2008. Taxonomic reclassification of Candida stellata strains reveals frequent occurrence of Candida zemplinina in wine fermentation. FEMS Yeast Res., 8, 328–336. [ Links ]
di Maro E., Ercolini D., Coppola S., 2007. Yeast dynamics spontaneous wine fermentation of the Catalanesca grape. Int. J. Microbiol., 117, 201-210. [ Links ]
Duarte F.L., Pimentel N.H., Teixeira A., Fonseca Á., 2012. Saccharomyces bacillaris is not a synonym of Candida stellata: reinstatement as Starmerella bacillaris comb. nov.. Antonie van Leeuwenhoek, 102, 653–658. [ Links ]
Esteve-Zarzoso B., Belloch C., Uruburu F., Querol A., 1999. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst.Bacteriol., 49, 329-337. [ Links ]
Fleet G.H., 2003. Yeast interactions and wine flavour. Int. J. Food Microbiol., 86, 11-22. [ Links ]
Hebenbrock K., Williams M.P., Karger L.B., 1995. Single-strand conformation polymorphism using capillary electrophoresis with two-dye laserinduced fluorescence detection. Electrophoresis, 16, 1429–1436. [ Links ]
Jolly N., Augustyn O., Pretorius I., 2006. The role and use of non-Saccharomyces yeasts in wine production. S. Afr. J. Enol. Vitic. 27,15-39. [ Links ]
Kurtzman C.P., Robnett C.J., 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek, 73, 331-371. [ Links ]
Lachance M-A., Boekhout T., Scorzetti G., Fell J.W., Kurtzman C.P., 2011. Candida Berkhout. In: The Yeasts, a taxonomic study. Vol 2, 987–1278. Kurtzman C.P., Fell J.W., Boekhout T. (eds), 5th edn. Elsevier, Amsterdam. [ Links ]
MacGregor B.J., Amann R., 2006. Single-stranded conformational polymorphism for separation of mixed rRNAS (rRNA-SSCP), a new method for profiling microbial communities. Syst. Appl. Microbiol., 29, 661–670. [ Links ]
Mounier J, Monnet C., Jacques N., Antoinette A., Irlinger F., 2009. Assessment of the microbial diversity at the surface of Livarot cheese using culture-dependent and independent approaches. Int. J. Food Microbiol., 133, 31–37. [ Links ]
Peters S., Koschinsky S.F., Schwieger C., Tebbe C., 2000. Succession of microbial communities during hot composting as detected by PCR–single-strand-conformation polymorphism-based genetic profiles of small-subunit rRNA genes. Appl. Environ. Microbiol., 66, 930–936. [ Links ]
Prakitchaiwattana, C.J., Fleet, G.H., Heard, G.M., 2004. Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res., 4, 865-877. [ Links ]
Renouf V., Claisse O., Lonvaud-Funel A., 2007. Inventory and monitoring of wine microbial consortia. Appl. Microbiol. Biotechnol., 75, 149-164. [ Links ]
Sampaio J.P., Gadanho M., Santos S., Duarte F.L., Pais C., Fonseca A. Fell J.W., 2001. Polyphasic taxonomy of the basidiomycetous yeast genus Rhodosporidium: Rhodosporidium kratochvilovae and related anamorphic species. Int. J. Syst. Evol. Microbiol., 51, 687-697. [ Links ]
Walsh T.J., Francesconi A., Kasai M., Chanock S.J., 1995. PCR and single-strand conformational polymorphism for recognition of medically important opportunistic fungi. J. Clin. Microbiol., 33, 3216-3220. [ Links ]
Wang Q.M., Li J., Wang S.A., Bai F.Y., 2008. Rapid differentiation of phenotypically similar yeast species by single-strand conformation polymorphism of ribosomal DNA. Appl. Environ. Microbiol., 74, 2604-2611. [ Links ]
Zanol G., Baleiras-Couto M.M., Duarte F.L., 2010. Restriction profiles of 26S rDNA as a molecular approach for wine yeasts identification. Ciência Téc. Vitiv., 25, 75-85. [ Links ]
*Corresponding author:
Tel.: +351 261712106; Fax: +351 261712426, E-mail: filomena.duarte@iniav.pt
(Manuscrito recebido em 23.11.2012. Aceite para publicação em 23.01.2013)













