Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciência e Técnica Vitivinícola
versão impressa ISSN 0254-0223
Ciência Téc. Vitiv. vol.28 no.1 Dois Portos jun. 2013
Comparison of two techniques for measuring leaf water potential in Vitis vinifera var. Albariño
Comparação de duas técnicas de medição do potencial da água na folha em Vitis vinifera var. Alvarinho
E. M. Martínez1, B. J. Rey1, M. Fandiño1, J. J. Cancela1*
1 GI-1716, Proyectos y Planificación. Dpto. Ingeniería Agroforestal. Universidad de Santiago de Compostela. Escuela Politécnica Superior, Campus Universitario s/n. 27002. Lugo (España).
*Corresponding author:
SUMMARY
The need to determine the variables that help characterize the real water status of vineyards calls for further research aimed at testing current techniques for water status quantification, and particularly for the determination of leaf water potential (LWP). The Scholander Pressure Chamber (SPC) has been widely used and considered as the reference technique. Yet, the frequent use of thermocouple psychrometers and, more specifically, of water activity meters (WAMs) demands a comparative analysis of the performance of SPC and WAMs and the applicability of WAMs to plant samples instead of soil samples, which has seldom been studied. This paper presents a comparison of two techniques for the determination of leaf water potential in Vitis vinifera var. Albariño in Galicia (NW Spain), WAMs (two models: WP4 and WP4-T) and SPC (model 600-PMS). In addition, this paper presents an assessment of the time required to perform determinations with the three instruments tested. The performances of the three instruments were assessed during two seasons: in 2011, we assessed SPC performance against WAM performance; in 2010, we assessed the performances of the two models of WAM. The comparison between SPC and WP4 was carried out by randomly selecting 22 vines under two irrigation treatments [rainfed (R) and irrigation] and with two irrigation systems [surface drip irrigation (DI) and subsurface drip irrigation (SDI)]. LWP readings were taken simultaneously with SPC and WAM model WP4. The performance of the two WAM models was assessed in terms of calibration protocol and measurement protocol. To this end, five vines were randomly selected and the following physiological indicators were determined: predawn and midday LWP, predawn and midday osmotic LWP and stem water potential. The time required for measurements was computed for the three instruments. Results reveal a strong correlation between LWP values measured with SPC and WAM (WP4 model), with coefficients of determination above 0.84. According to the results, WAMs are more versatile than SPC, but SPC measurements require less time. Among WAMs, WP4 produces faster measurements than WP4-T and requires fewer calibrations per sample. The use of a WP4 WAM to take real measurements produced reliable results and allowed for the determination of plant water status according to different irrigation treatments showing high sensitivity to plant water status variations among treatments.
Key words: correlation, water activity meter, Scholander pressure chamber, fertigation.
RESUMO
A necessidade de determinação das variáveis que permitam a caracterização do estado de hidratação real das videiras requer mais investigação, nomeadamente no teste das atuais técnicas de quantificação do estado da água e, em particular, as de determinação do potencial hídrico das folhas (LWP). A câmara de pressão de Scholander (SPC) tem sido amplamente utilizada e é considerada como técnica de referência. No entanto, o uso frequente de psicrómetros de termopares e, mais especificamente, de medidores de atividade da água (WAMs) torna necessário a análise comparativa da eficiência dos SPC e WAMs e da aplicabilidade destes últimos a amostras de plantas, em alternativa a amostras de solo. Contudo, a aplicabilidade de WAMs para medições de amostras de plantas tem sido raramente estudada. Este estudo apresenta a comparação de duas técnicas de determinação do potencial hídrico das folhas em Vitis vinifera var. Alvarinho na Galiza (NO Espanha), WAMs (WP4, WP4-T) e SPC (600-PMS), apresentando uma avaliação do tempo necessário nas determinações para os três aparelhos. O desempenho destes tês instrumentos foi avaliado durantes duas campanhas: em 2011, foi avaliado o desempenho da SPC em comparação com o dos WAM; em 2010, avaliou-se o desempenho de dois modelos de WAM. A comparação entre SPC eWP4 foi efetuada através da escolha aleatória de 22 vinhas submetidas a dois tratamentos de rega [sequeiro (R) e regada] e dois sistemas de rega [microrrega superficial (DI) e enterrada (SDI)]. As leituras de LWP foram efetuadas em simultâneo com a SPC e o modelo WP4. O desempenho dos dois modelos de WAM foi avaliado em termos de protocolo de calibração e de medição. Para tal, foram escolhidas cinco vinhas ao acaso e foram determinados os respetivos indicadores fisiológicos: LWP pré-amanhecer e a meio do dia, LWP osmótico pré-amanhecer e a meio do dia e potencial de ramo. O tempo necessário para as medições foi estimado para os três instrumentos. Os resultados demonstram uma forte correlação entre os valores de LWP medidos com SPC e WAM (modelo WP4), com coeficientes de determinação superiores a 0,84. De acordo com os resultados, os WAMs são mais versáteis do que os SPC, mas as medições com SPC requerem menos tempo. Entre os WAMs, os WP4 produzem medições mais rápidas do que os WP4-T e requerem menos calibrações por amostra. O uso de um WP4 WAM produziu resultados fiáveis e permitiu a determinação do estado hídrico da folha consoante os diferentes tratamentos de rega, devido à sua sensibilidade às variações entre tratamentos.
Palavras-chave: correlação, medidores de atividade da água, câmara de pressão de Scholander, fertirrega.
INTRODUCTION
In terroirs, water is the key to growth and production, both quantitatively and qualitatively (Bravdo and Naor, 1997; Chapman et al., 2005; Ferreyra et al., 2006). Vine water status is strongly influenced by the soil and the meteorological conditions (Van Zyl, 1987; Barbeau et al., 2004), insofar as vines obtain water by rainfall or irrigation (Deloire et al., 2004). When natural water availability is low, an efficient irrigation strategy should supply just enough water to achieve the yield and juice composition required by the winery (Green et al., 2003). Grapevine irrigation is a highly controversial issue (Nadal and Lampreave, 2004), but the response of grapevines to heat and drought is different when irrigated (Chalmers et al., 2007). As pointed out by Petrie et al. (2004), recent improvements in irrigation techniques such as drip irrigation (Roby et al., 2004), regulated deficit irrigation (Ghaderi et al., 2007, Santesteban et al., 2007) or partial rootzone drying (Intrigliolo et al., 2007a) have improved water use efficiency and allowed for the extension of viticulture beyond areas with natural water supply.
The effects of irrigation have been widely studied (Naor et al., 1993, Nadal and Arola, 1995; Giorgessi et al., 1998, Ferreyra et al., 2002; Walker et al., 2002; Santalucia et al., 2007). Generally, water stress is required in grapevines to obtain maximum fruit quality (Jackson and Lombart, 1993). Medrano et al. (2007) claimed that water deficit is not easy to determine because of the large number of variables involved, among which variety-pattern, phenology, soil and climate conditions, number of clusters, berry size or concentration of components, which allow for the definition of the relationship between harvest quality and level of water deficit.
According to Van Leeuwen et al. (2010), the techniques for measuring vine water status can be classified into three approaches according to the principle of measurement used: measurement of soil water, measurements of physiological indicators or water balance modelling, which are complementary, robust and easy to implement. Among physiological indicators are transpiration, water potential, microvariations in stem or berry diameter, differences between leaf and air temperatures, carbon isotope discrimination measured on grape sugars, sap flow measurements and growth parameters. Consequently, vines can be used as indicators of their own water status and provide information on physiological indicators (Hidalgo, 2006), which can be used as indicators of water stress (Yuste et al., 2004).
Botella and Campos (2005) suggested four approaches to determine water potential: liquid compensation methods, gas phase compensation methods, psychrometric methods (thermocouple psychrometers-TCPs) (Mullins, 2001) and pressure chamber methods (Scholander et al., 1965). Today, pressure chamber methods are the most used methods to measure leaf water potential (LWP).
SPC provides a measure of the negative hydrostatic pressure that occurs in the xylem of an intact plant because of: i) water evaporation from the tissue by transpiration and ii) resistance to water movement from soil to tissue. Accordingly, LWP has been estimated as the negative pressure value required to obtain liquid on the surface of the xylem exposed to atmospheric pressure (Scholander et al., 1965; Campbell, 1985; Busso, 2008). A number of authors have reported the benefits of SPC as a simple and rapid method that is highly useful under field conditions (Boyer, 1967; Afonso et al., 2003; Carbonneau and Costanza, 2004; Kirkham, 2005; Lopes et al., 2008; Van Leeuwen et al., 2009). Actually, SPC has been considered an ideal instrument for measurement of the water potential of foliage in ecological, physiological and agronomic studies (Ritchie and Hinckley, 1975; Acevedo-Opazo et al., 2013; Escalona et al., 2013). Yet, Millar and Hansen (1975), Turner and Long (1980), and Phillips (1981) found potential SPC measurement errors in plant material and complications with end-point recognition at which the balancing pressure equals the xylem pressure potential, which is difficult to determine (Phillips, 1981) because it is dependent on operator, leaf age at measurement time, cut type and measurement boundary conditions, which can entail visualization problems. Améglio et al. (1999) pointed to the limitations of SPC to determine LWP as an indicator of stress in conditions of heterogeneous soil humidity. As claimed by Busso (2008), among other authors, SPC is a convenient, accurate and reliable method for measuring water potential, as long as good techniques are used and the appropriate precautions are taken. Pire et al. (1988) performed an inverse calibration of the SPC using a TCP, specifically a thermocouple hygrometer, which has been considered potentially more accurate (Busso, 2008). The literature review conducted suggests that SPC and TCPs are the most suitable instruments for measuring LWP; hence the need to validate WAMs as a subgroup of TCPs.
TCPs allow for continuous measurements of leaf water potential (McBurney and Costigan, 1987) and are considered the only non-destructive method available (Martínez et al., 2011). In New World viticulture (Australia, USA, among others), both irrigation consultants and large vineyards assess plant water needs by determining vine water status from leaf water potential (Intrigliolo et al., 2007b), which allows for monitoring of the water relations of grapevines (Smart and Coombe, 1983; Williams and Araujo, 2002). Some techniques enable continuous monitoring of vine water status and, consequently, of the irrigation scheduling scheme systems used in vineyards. Such techniques are based on multispectral aerial imagery using unmanned aerial vehicles (UAV) (Johnson et al., 2003) and on the modelling of the evolution of soil water content in the root zone (Fandiño et al., 2012) and its relationship with leaf water potential.
In the 1990s, a new subgroup of psychrometric techniques that were considered a type of TCPs within dewpoint meters emerged (Mullins, 2001, Livingston and Topp, 2006). The new techniques involved the use of water activity meters (WAMs) (Gee et al., 1992), which were presented as a versatile tool for analysing samples in terms of their state (liquid or solid), type (soil or plant, among others) (Díaz-Zorita et al., 2004; Rivero et al., 2007; Padilla et al., 2009), location, collection method (roots, stems, leaves, seeds, fruits) and treatment (disturbed or undisturbed samples) (Petry and Jiang, 2003; Cancela, 2004). A number of authors verified the suitability of WAMs for performing measurements in soil samples as compared to the reference methods (Cancela et al., 2006) by analysing the factors that affected the measurement process (Martínez and Cancela, 2011) and tested their applicability under different conditions.
For plant measurements, WAMs are not so commonly used but they have been used for small applications in some countries like China, Japan, Italy, India, Belgium, France or Spain (Londers et al., 2005; Lechaudel et al., 2007; Maggio et al., 2007; Patel and Pandey, 2007; Xu and Zhou, 2008; Kondo et al., 2009; Martínez et al., 2011). Accordingly, further studies are needed to support the use of WAMs in plants and define a measurement method that has been contrasted with the reference method.
This paper aims to assess two techniques for the determination of leaf water potential (LWP) in Vitis vinifera var. Albariño according to the following steps: i) assessment of water activity meters (WAM) and comparison with Scholander pressure chamber (SPC), which has been considered the reference method; ii) comparison of two types of water activity meters (with or without control of measurement temperature) for the determination of leaf water potential; and iii) analysis of the time required with each measurement technique and instrument.
MATERIAL AND METHODS
Description of the area and experimental design
Research was conducted in two seasons: 2010 and 2011 in a commercial Albariño vineyard located at Porto, Salvaterra do Miño, in the Condado do Tea wine-producing sub-region of Rías Baixas, Pontevedra, NW of Spain (42°3.5´N, 8°32.2´W). The study area enjoys a subhumid microclimate with an Atlantic influence, characterized by mild temperatures and high rainfall during the autumn and spring months, with Koppen classification Cfb (Peel et al., 2007). Data from two nearby agricultural weather stations, Entenza and Meder, were used to characterize the meteorological conditions of the area during the period of analysis, from March to September.
The selected vineyard was terraced and vines were planted in rows. The experimental design included two terraces with four and five rows of vines, respectively. Vines were planted on 19617C rootstocks at 1.5 m spacing within the row and 3.0 m spacing between rows, running mainly in a North-South direction. Yet, some plots were planted in a Northwest-Southeast direction. A high training system was used (1.5 m above ground), with long pruning and vigorous vines, which were arranged in an inclined semi-trellised system (Hidalgo, 1993) with four wires and spur-pruned to the Guyot system.
A block design was used, with a stratified simple random sampling to select the homogeneous group in each block, using the sanitary status of wines. The experimental study comprised the following treatments and irrigation systems:
• rainfed (R);
• surface drip irrigation (DI) using drippers with a flow rate of 2 L.h-1 per plant located 30 cm above the ground;
• subsurface drip irrigation (SDI) using pressure compensating drippers with a flow rate of 2 L.h-1 buried at 30 cm depth.
Both DI and SDI systems were used to apply nutrients (N, P, K and Mg) at a rate of 0.44-0.66 mm. d-1, which was scheduled as a 1.5 h application five days a week from May 4 to August 31 in 2010, and April 13 to August 17 in 2011, because the goal was fertigation and regulated deficit irrigation.
During 2010, five vines under the considered treatments were randomly selected: two vines under SDI, one vine under R in the first terrace, and one vine with DI and R in a second terrace.
Field and laboratory analysis techniques
To determine LWP with both techniques, healthy mature leaves from the middle third of the shoot were sampled, all with similar growth stages and no alterations, and exposed to direct solar radiation (Maringo and Peltier, 1996). Leaves were covered with a plastic bag and petioles were cut. The samples were stored in an ice-box at low temperatures and near 100% relative humidity, and transported to the laboratory under controlled conditions in the shortest possible time (Ferreyra et al., 2007), about 3-5 minutes. For covered leaves used for the determination of stem water potential, leaves were enclosed in black polyethylene bags shielded with aluminium foil for at least two hours before excision (Turner, 1981).
Scholander pressure chamber
The SPC used for determinations was a model 600 Pressure Chamber Instrument (PMS Instrument Company).
During the 2011 season, measurements made with WAM model WP4 were validated. SPC measurements of LWP were taken at different times of the day in 22 vines. Three readings were taken per sample, such that the total number of LWP readings was 66. LWP measurements with WP4 were taken simultaneously. Finally, the times required by both instruments to complete the process were computed.
SPC measurements were performed according to the following method (Boyer, 1967; Intrigliolo et al., 2007b): leaves were covered with a plastic bag and the petiole was cut using a blade. Then each leave with bag was sealed in the chamber such that the cut end of the petiole was exposed outside the chamber and pressure was applied to the leaf with nitrogen until sap appeared at the cut surface. To avoid potential handling errors, the chamber was previously adjusted and measurements were performed by qualified staff.
Water activity meters
LWP measurements were made with two WAM models, WP4 and WP4-T (Decagon Device, Inc.). Both WP4 and WP4-T are based on the chilled mirror dewpoint technique (Gee et al., 1992; Mullins, 2001; Scanlon et al., 2002; Martínez et al., 2011). Consequently, the sample is equilibrated with the headspace of a sealed chamber that contains a mirror and means of detecting condensation on the mirror. At equilibrium, the water potential of the air in the chamber is equivalent to the water potential of the sample. The main difference between both models regards temperature control: model WP4 does not include temperature control, such that sample temperature is affected by boundary conditions, whereas model WP4-T is a user-selectable internal temperature control model that uses thermoelectric (Peltier) components (Scanlon et al., 2002; Fredlund et al., 2012) to maintain a constant internal temperature pre-set by the user. Scanlon et al. (2002) pointed to the importance of appropriate temperature control. Thus, to attain ± 0.1 MPa accuracy, the measured difference between dewpoint and sample temperature must be accurate to ± 0.005 °C, which is most readily attained when the sample and chamber temperatures are about the same (within ± 0.5 °C). For WAMs, the temperature difference between the sample and the block must be less than 0.1 ºC before starting water potential measurements (Decagon Device, 2000).
In both models, the appropriate calibration and measurement protocols must be defined. In our research, both instruments were calibrated according to the procedure suggested by Martínez and Cancela (2009) and to the recommendations made by Decagon Devices and authors such as Fredlund et al. (2012), among others. Accordingly, the instruments were calibrated between samples with KCl solution (0.5 M). Calibration was repeated four times and the arithmetic mean of the four calibrations was considered. To verify the validity of the calibration protocol, 10 continuous readings of the same calibration solution of known potential (KCl 0.5 M; Ψ=-2.19 MPa) were taken, which allowed for the determination of the best moment to calibrate the instruments based on the number of consecutive readings that could be performed without obtaining a deviation of measured values from real values above 0.10 MPa, which was the accuracy of the instruments.
Once the calibration procedures required for both models were defined, the physiological indicators, predawn (Ψp) and midday (Ψm) leaf water potential, midday stem water potential (Ψs) and predawn (Ψop) and midday (Ψom) osmotic potential were determined in five vines under different irrigation treatments and irrigation systems (R, DI and SDI) for six days (five days for predawn LWP) throughout the 2010 season, which resulted in 120 readings for each indicator with each WAM model (WP4 and WP4-T). For WAM assessment, LWP measurements were made during the period of maximum vineyard water requirements, from June to August (pre-harvest), and in September (post-harvest). Measurements were performed after overnight rehydration (predawn LWP values) (Richter, 1976, Itier et al., 1990, Tardieu et al., 1991, Katerji et al., 2000) and under maximum stress (midday LWP values) (Girona et al., 2006), both in exposed and covered leaves (leaf or stem potential) (Choné et al., 2001). Predawn measurements were performed before dawn, from 05:30 to 08:00, and midday measurements were performed at solar noon, between 11:00 and 15:00. In addition, because plants show osmotic adjustment with variations caused by periodic water deficits (O´Neill, 1983), we measured leaf osmotic potential, which was considered a physiological indicator dependent on plant water status. Leaf osmotic potential was measured according to the following method: samples were frozen by immersion in liquid nitrogen for 4 minutes and later thawed and placed into sealed chambers, avoiding leaf dehydration by contact with the refrigerating medium.
Measurements were made in leaf discs of 1.2 cm diameter (Londers et al., 2005). Ten discs were punched with hole punch pliers (Barrs, 1968) after leaf cuticle removal by abrasion (Campbell and McInnes, 1999). Discs were arranged such that the sample cup was fully covered and the measurement process was unaltered. Measurements were performed under laboratory conditions: for model WP4 measurements, the temperature of the readings was dependent on sample and chamber temperature, whereas for WP4-T measurements, temperature was set at 25 ºC, which was the average temperature recorded for the same period of the previous season. Four repetitions were performed and the arithmetic mean of the four readings was considered as the leaf water potential value. Readings were taken simultaneously with both instruments in order to minimize the potential differences caused by variability in boundary conditions.
Additionally, the times involved in the measurement process were computed, namely: temperature equilibration time (Te), read time (Tl) and total read time (Tt). According to Martínez (2008), Te is the time during which the instrument adjusts the temperature of the sample to match the temperature inside the chamber, Tl computes the time required to obtain the value of the measured potential after several readings, and Tt is the sum of Te and Tl.
In this paper, the repeatability of WAM measurements has been verified through comparison with SPC as the reference method.
Indicators of measurement accuracy
The measurement accuracy of the instruments was assessed using mainly two approaches: first, by graphically comparing WP4 and WP4-T values with SPC values, which allowed us to find measurement trends or biases; second, by calculating the linear regression between WAM and SPC values.
In addition, the following indicators of measurement error were used (Cholpankulov et al., 2008):
The root mean square error (RMSE), which characterizes the variance of the errors and is expressed in the same units as the observed values Oi:
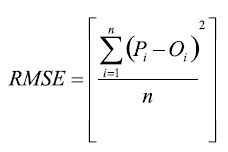
(1)
where Oi and Pi (i=1, 2, , n) are the pairs of water potential values obtained with the analysed instruments.
The average absolute error (AAE), which expresses the size of estimation errors as an alternative to RMSE in the same units as Oi:
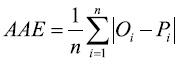
(2)
The average relative error (ARE), which indicates the size of errors in relative terms and is expressed as a percentage:
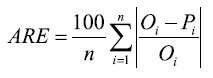
(3)
The maximum absolute error (Emax), which is expressed in the same units as Oi:
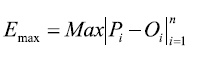
(4)
For the statistical analysis of differences between treatments, a Duncan multiple range test was performed using SPSS v.19.
RESULTS AND DISCUSSION
Comparison of WAM model WP4 and SPC
Results reveal an underestimation of leaf water potential values for WP4 (more negative values of water potential) as compared to SPC, with a mean of 0.43 ± 0.06 MPa, a maximum difference of 0.56 MPa and a minimum difference of 0.31 MPa (Fig. 1). The fit of the regression line produces a coefficient of determination (r2) of 0.84.
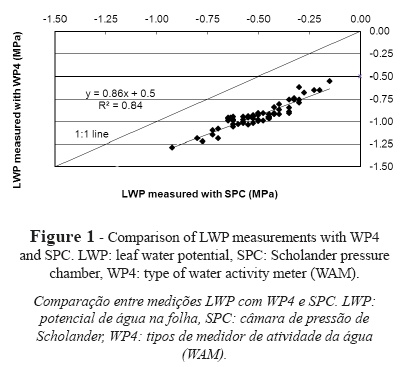
A number of authors have compared measurements of water potential performed with SPC and other techniques. Boyer (1967), Duniway (1971), Brown and Tanner (1981), Kikuta et al. (1985) and Turner et al., (2000) compared thermocouple psychrometers (TCP) and SPC in plant samples. A comparison of SPC and leaf-cutter psychrometers, which used leaf discs for LWP measurements, showed more dehydrated values of leaf water potential for leaf-cutter psychrometers when tissue was damaged, with differences of up to 0.2 MPa. Yet, the results obtained for both techniques were similar for measurements under non-transpiring conditions (Turner et al., 2000). Brown and Tanner (1981) reported similar results for both techniques in tests conducted in alfalfa, but obtained differences above 0.2 MPa in fully-exposed plants. In a study performed in tomato plants, Duniway (1971) suggested two factors that explain LWP underestimation in TCP measurements: i) psychrometric techniques involve leaf excision, which results in water loss to a greater or lesser extent depending on the volume of xylem in the leaf blade, ii) movement of water from xylem to mesophyll cells. Boyer (1967) pointed to the effects of the species used for analysis. Actually, Boyer reported variations in LWP measurements with TCP as compared to LWP measurements with a balancing SCP of ± 0.2 MPa for sunflower and yew. For rhododendron, LWP values measured with SPC were between 0.25 MPa less negative and 0.4 MPa more negative than LWP values measured with TCP. Similarly, Kikuta et al. (1985) reported variations of LWP measured with a leaf hygrometer and an SPC in the range 0.01-0.25 MPa according to species.
Given the similarity of the protocol suggested in this paper to the protocol followed with the most traditional TCPs, we have assumed that there is implicit variation in the protocol, in agreement with Barrs (1964), Boyer and Knipling (1965), Klepper and Barrs (1968), among others. Our results revealed variations between WAMs when disc samples were used, which involved cell rupture and tissue damage and a potential loss of water from the tissue. In contrast, SPC used intact leaf tissue for LWP measurements. Thakur et al. (2006) reported underestimations of 1.1 MPa for LWP values measured with WAM in liquid samples of known potential as compared to tabulated data. On the contrary, Cancela et al. (2006) reported overestimations of LWP values measured with WAM in soil samples as compared to Richards pressure plate measurements (Murray and Sivakumar, 2010). The measurement variability of WAMs is intrinsic to the measurement procedure of the instrument and is dependent on the conditions of the headspace above the sample in the reading chamber. Consequently, some variations are caused by the operation of the instrument and some variations are caused by the calibration and measurement processes. Yet, the intrinsic effect of the instrument is counterbalanced by appropriate monitoring of measurement conditions and appropriate calibration of each instrument.
The results presented in this section, with different LWP values measured with both SPC and WAM, allow for appropriate determination of real crop stress at any time during measurement. The difference between SPC and WAM measurements was in the range 0.31-0.56 MPa. WAM-measured values were more negative, which could be associated with unreal grapevine water stress and lead to inappropriate irrigation practices. Authors such as Carbonneau (1998), Deloire et al. (2003) or Zufferey (2007) established the level of grapevine stress based on predawn LWP thresholds determined with SPC, which could be wrongly interpreted if measured with WAMs. According to this relationship, LWP measurements obtained with WAMs allow us to determine the real water status of the plant and the presence or absence of grapevine stress. This is particularly relevant when SPC cannot be used, e.g. to determine osmotic potential or in parts of the plant where SPC cannot be applied, such as fruits or seeds. In these cases, WAMs are particularly useful because of their versatility in terms of the types of materials that can be measured, as claimed by Martínez et al. (2011).
Analysis of WAM data
During calibration, repeated measurements of the water potential of the KCl solution revealed mean errors of 0.09 ± 0.05 MPa for model WP4 and 0.12 ± 0.06 MPa for model WP4-T. The mean error for model WP4-T exceeds the accuracy of the instrument (Table I). Both instruments show a tendency to loss of calibration: model WP4 increasingly overestimates the value of water potential (less negative), whereas model WP4-T shows a fluctuating tendency. For this reason, model WP4-T requires two calibrations per sample (four readings/iterations per sample with calibration every two readings) and model WP4 requires only one calibration (calibration every four readings). This represents a definite drawback for model WP4-T as compared to model WP4, despite WP4-T being further evolved. Yet, WP4-T must be used for measurements that must be performed under a stable pre-set temperature, because this is the only model whose design allows for this feature.
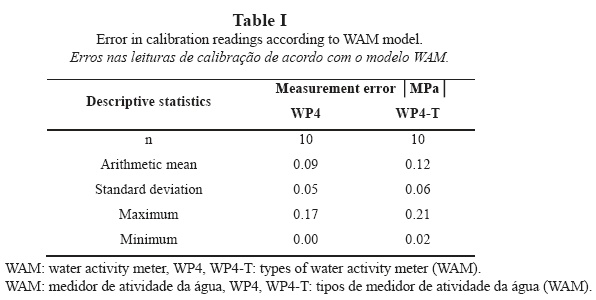
Model WP4-T provides repeated values of water potential, with minimum variations between readings and samples that are below the accuracy of the instrument for 79% of the measurements. For model WP4, measurement repeatability increases up to 83%.
For measurements of physiological indicators, a good fit was obtained by using the set of water potential values obtained from simultaneous measurements with WP4 and WP4-T in the same samples for all the indicators (Fig. 2). The statistical analysis performed considering WP4 as the reference technique yielded a coefficient of determination of 0.96, an RMSE of 0.055 MPa, an AAE of 0.051 MPa, an ARE of 4.79 MPa, and a maximum absolute error (Emax) of 0.19 MPa. The relationship between both instruments shows a slight underestimation of WP4-T water potential values as compared to WP4 values and, therefore, WP4-T readings were more negative.
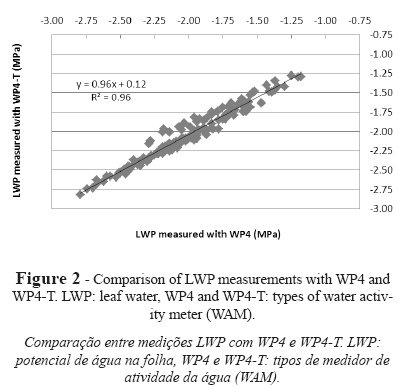
The use of WP4 instead of WP4-T as the reference technique is supported by previous research conducted by Cancela et al. (2006). Measurements made with WP4 and WP4-T are dependent on correct calibration. In this sense, the calibration protocol proposed by Martínez and Cancela (2009) can be considered as a reference method because it involves the use of calibration solutions of known molarity for both instruments. The two models of WAM used in this analysis work differently in terms of calibration. As explained earlier in this paper, both models use the same type of calibration but require different calibration frequencies. During calibration with solutions of known molarity, WP4 enables four consecutive readings without exceeding the allowable upper limit whereas WP4-T enables only two consecutive readings. This suggests that calibration must be handled differently according to the instrument involved. For measurements in soil samples, Cardoso et al. (2007) questioned the convenience of using the calibration protocol recommended by the manufacturer of the equipment, which was a WP4 WAM.
Following the appropriate calibration process, WP4-T took reliable measurements but underestimated LWP readings (more negative values), which should be taken into consideration by making the relevant corrections of the readings. Martínez (2008) assessed the performance of WP4-T and WP4 and found that WP4-T measurements were not repeatable. She claimed that the causes for measurement unrepeatability with WP4-T were the unsuitability of the calibration protocol suggested by the manufacturer of the instrument, as verified in the assessment presented in this paper, and the effects of the boundary conditions on the readings caused by the dependence on Kelvins equation of some environmental factors such as temperature and relative humidity. The effects of such factors were reported also by Thakur et al. (2006), Cardoso et al., (2007), Martínez et al., (2007) and Martínez and Cancela (2011). However, because our research was conducted in laboratory conditions, these factors had no effects on measurements. Therefore, the variations found in the potentials measured with both WAM models could be due to the measurement process and the differences in the measurement temperature considered in both instruments, which was 25 ºC for WP4-T and fluctuating with ambient conditions for WP4.
Figure 3 shows the results for the evolution of the physiological indicators as measured with both instruments. For LWP, some differences were observed between irrigation treatments and systems during the season, such that the mean potential values obtained with WP4 for vines under R (-2.14 MPa) were lower than the values obtained for than vines under SDI (-1.77 MPa) or DI (-2.02 MPa), which suggests a lower water status in vines under R. Vines under R showed mean potential values of -1.64 MPa at early measurements, which evolved to -2.48 during the post-harvest. Irrigated vines evolved from -1.31 MPa under SDI and -1.58 MPa under DI in June to -2.07 MPa and -2.37 MPa, respectively, at the end of the season.
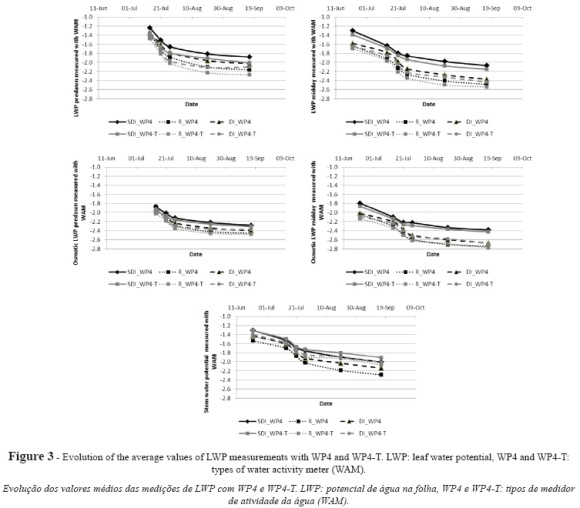
For WP4-T measurements, the mean values were -2.21 MPa for vines under R, -2.21 MPa for vines under SDI and -2.08 MPa for vines under DI. Similar differences between both instruments were obtained for the rest of indicators (Fig. 3). With WP4-T, initial mean values were -1.70 MPa for vines under R, -1.39 for vines under SDI and -1.64 MPa for vines under DI, and post-harvest values were -2.54 MPa for R, -2.15 MPa for SDI and -2.43 MPa for DI.
Table II shows the mean values obtained with WP4 for the physiological indicators according to treatment and irrigation system. Overall, the five indicators confirm a higher water status in vines under SDI as compared to DI, and the highest stress levels in rainfed vines (R). The observed differences are statistically significant for midday measurements of LWP and osmotic leaf water potential.
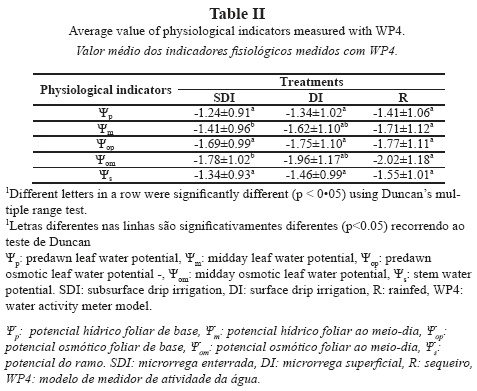
Analysis of measurement times
Some stages of the process, such as the extraction/collection of samples from vines, are common to the three measurement techniques tested. Because the time required for sample collection is considered equivalent for all three techniques, sampling time was not included in the calculation of the time required to complete measurements. Likewise, to simplify the methods, the same number of readings per sample (number of iterations) was considered for the three instruments. Conversely, calibration time varies according to the instrument. For SPC, it has been assumed that the instrument does not require calibration because it has already been tested. For WAMs, each model requires a different calibration process, insofar as model WP4-T requires two calibrations per sample and WP4 requires only one calibration per sample, as suggested in the analysis of WAM data section.
For WAM calibration (Fig. 4a), average temperature equilibration time (Te avg) was 2.57 ± 0.30 min for WP4 and 4.46 ± 1.11 min for WP4-T. WP4-T measurements were taken at a pre-set temperature and WP4 measurements were made at ambient temperature. Consequently, equilibrating temperature took longer with WP4-T, with a difference in Te values between both instruments of 1.89 min, which accounted for a Te increase of 75% in measurements performed at a stable temperature. For WP4, average Tl amounted to 2.66 ± 0.40 min, whereas for WP4-T, average Tl increased by 32% (3.51 ± 0.98 min). As a result, the total time required to complete the calibration process (Tt) amounted to 5.23 min for WP4 and 7.97 min for WP4-T.
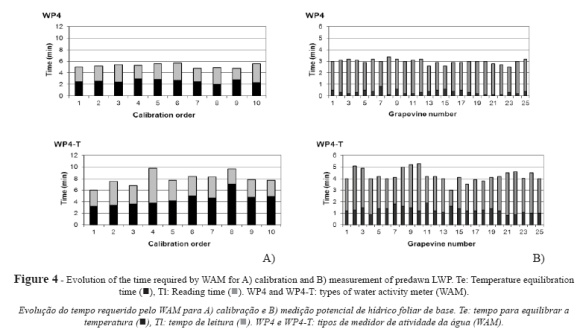
The results for predawn LWP measurements conducted in five vines for five days in a season (25 samples and 100 readings) using correctly calibrated instruments (Fig. 4b) and considering the average of the time required to take four readings per sample can be extrapolated to the rest of the potentials determined. The following values were obtained for the time required to perform LWP measurements with WP4: Te = 0.31 ± 0.18 min, Tl = 2.64 ± 0.30 min and Tt = 2.95 ± 0.22 min. As compared to the time required for calibration, these values involved 88%, 1% and 44% decreases for Te, Tl and Tt, respectively. The time obtained from simultaneous measurements with WP4-T are Te = 1.23 ± 0.28 min, Tl = 3.02 ± 0.56 min and Tt = 4.25 ± 0.49 min. With respect to calibration time, measurement time decreased by 72% for Te, 14% for Tl and 47% for Tt.
The variables related to osmotic potential cannot be determined using SPC. Determination of LWP required 11.02 hours with WP4 (Table III) and 18.97 hours with WP4-T, which increased by 72.1% the time required by WP4.
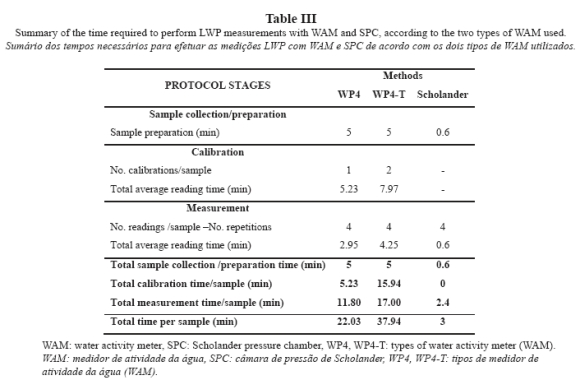
If SPC were applied to the same vines, the times required by SPC would be substantially shorter than the times required by both WAMs (WP4 and WP4-T), with 86% decrease as compared to WP4 and 92% decrease as compared to WP4-T. During the whole season, only 1.5 hours would be required by SPC to perform measurements in five vines during six days taking four readings (for consistency with WAMs), per parameter.
Variations in the time required for calibration and measurement are intrinsically related to the nature of the sample; liquid for calibration (a solution of known potential) and solid for measurements (leaves). Even within the same type of sample, variations were found in the time required to complete the process with respect to the time reported by Cancela et al. (2006), Martínez (2008) and Martínez and Cancela (2009): an average Tt of 10.33 min for calibration solutions and an average Tt of 4.5 min per sample for soil samples. The different nature of the sample can delay or advance the achievement of dynamic equilibrium between the gas phase contained in the headspace of the reading chamber and the liquid phase contained in the measured sample. In case that the SPC technique, which is much faster and simpler, is not used, the use of WP4 is indicated because WP4 is less demanding and faster than WP4-T.
CONCLUSIONS
This paper reveals that leaf water potential determination in vineyards can be affected by the measurement instruments used, as confirmed by the results of the assessment of the performance of the two instruments currently used to determine LWP: SPC and WAMs. Regardless of the WAM model used, WP4 or WP4-T, leaf water potential readings provide underestimations of the value of LWP as compared to SPC. Consequently, if a relationship between both instruments is not established, measurements could be associated with grapevine stress levels in plants with adequate water status. Accordingly, the stress thresholds provided by the literature must be revised and new scales must be defined according to the measurement instrument used.
In this paper, WAMs are presented as instruments that require a detailed calibration protocol that is specific to each model as opposed to the simplicity of SPC, which does not require calibration. Both WAM and SPC follow a destructive measurement protocol, which is more time-demanding and laborious in terms of sample preparation for WAMs, particularly model WP4-T. According to results, WAMs are preferred for LWP determination insofar as, despite the mentioned drawbacks, WAMs allow for measurements at vine level that could not be performed with SPC, such as osmotic potential determination of water potential in plant parts other than leaves, such as the stem (using extracted plant material), fruits or seeds. Moreover, only WAMs and particularly WP4-T are capable of performing measurements at a constant temperature or under stable measurement conditions. Accordingly, operators must choose the most suitable instrument based on the characteristics of the measurement process, rapidity for SPC, or versatility in uses and stability of measurement conditions for WAMs. The analysis of the results and the determination of the presence of water stress or non-stress in vines will depend on the instrument used, considering the relationship between both instruments that has been defined in this paper. A strong correlation was found between LWP values measured with SPC and WAM, with coefficients of determination above 0.84.
Using WP4 to determine the indices of water stress in a commercial vineyard yielded significant differences between the assessed irrigation treatments and systems, with a higher water status in vines under surface drip irrigation (DI), as compared to rainfed vines (R). Vines under subsurface drip irrigation (SDI) showed the highest water status. New tests must assess and establish the stress thresholds in Vitis vinifera according to variety and variety-rootstock for determination with WAMs.
ACKNOWLEDGMENTS
This research has been partially funded by Consellería de Educación e Ordenación Universitaria-Xunta de Galicia and research program Isabel Barreto 2009. The authors are grateful to Dr Giovanni Pardinni and Dr Julián García, who provided them with the equipment, to the technical and administrative staff of Pazo San Mauro winery, and to technological centre Centro Tecnológico para el Desarrollo Industrial - Ministerio de Ciencia e Innovación, Spain.
REFERENCES
Acevedo-Opazo C., Valdés-Gómez H., Taylor J.A., Avalo A., Verdugo-Vásqueza N. Araya M., Jara-Rojas F., Tisseyre B., 2013. Assessment of an empirical spatial prediction model of vine water status for irrigation management in a grapevine field. Agr. Water Manage., 124, 58-68. [ Links ]
Afonso J.M., Monteiro A.M., Lopes C.M., Lourenço J., 2003. Enrelvamento do solo em vinha na região dos vinhos verdes. Três anos de estudo na casta Alvarinho. Ciência Téc. Vitiv., 18, 47-63. [ Links ]
Améglio T., Archer P., Cohen M., Valancogne C., Daudet F.A., Dayau S., Cruiziat P., 1999. Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil, 207, 155-167. [ Links ]
Barbeau G., Bournand S., Champenois R., Bouvet M.H., Blin A., Cosneau M., 2004. Comportement de quatre cépages rouges du Val de Loire en fonction des variables climatiques. J Int Sci Vigne Vin, 38, 35-40. [ Links ]
Barrs H.D., 1964. Heat of respirations as a possible cause of error in the estimation by psychrometric methods of water potential in plant tissue. Nature, 203, 1136-1137. [ Links ]
Barrs H.D., 1968. Determination of water deficits in plant tissues. In: Water deficits and plant growth. Vol. I. Development control and measurements., 235-368. Kozlowski T.T. (ed), Academic Press, New York. [ Links ]
Botella O., Campos I., 2005. Las relaciones agua-planta. In: Agua y Agronomía. 87-161. Martín F., López P., Calera A. (eds), Ed. Mundi-Prensa, Madrid. [ Links ]
Boyer J.S., 1967. Leaf water potentials measured with a pressure chamber. Plant Physiol., 42, 133-137. [ Links ]
Boyer J.S., Knipling E.B., 1965. Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proc. Nat. Acad. Sci. USA, 54, 1044-1051. [ Links ]
Bravdo B., Naor A., 1997. Effect of water regime on productivity and quality of fruit and wine. Acta Hortic., 427, 15-26. [ Links ]
Brown P.W., Tanner C.B., 1981. Alfalfa water potential measurements: a comparison of the pressure chamber and leaf dew-point hygrometers. Crop Sci., 21, 240-244. [ Links ]
Busso C.A., 2008. Uso de la cámara de presión y los psicrómetros a termocupla en la determinación de las relaciones hídricas en tejidos vegetales. Int. J. Exp. Bot., 77, 327-350. [ Links ]
Cancela J.J., 2004. Gestión integrada del agua en la Cuenca alta del río Miño. 466 p. PhD Thesis, Universidad de Santiago de Compostela. [ Links ]
Cancela J.J., Dafonte J., Martínez E.M., Cuesta T.S., Neira X.X., 2006. Assessment of a water activity meter for rapid measurements of soil water potential. Biosyst Eng., 94, 285-295. [ Links ]
Campbell C.S., McInnes K.J., 1999. Response of in situ leaf psychrometer to cuticle removal by abrasion. Agron. J., 91, 859-862. [ Links ]
Campbell G.S., 1985. Instruments for measuring plant water potential and its components. In: Instrumentation for environmental physiology. 193-214. Marshall B., Wodward F.I. (eds.), University Press, Cambridge, England. [ Links ]
Carbonneau A., 1998. Aspects in qualitatifs. In: Traté d´irrigation. 258-276. Tiercelin J.R. (ed.), Tec et Doc Lavoisier, Paris. [ Links ]
Carbonneau A., Costanza P., 2004. Response of vine leaf water potential to quick variation in canopy exposure. Example of canopy opening manipulation of Merlot (Vitis vinifera L.). J. Int. Sci. Vigne Vin, 38, 27-33. [ Links ]
Cardoso R., Romero E., Lima A., Ferrari A., 2007. A comparative study of soil suction measurement using two different high-range psychrometers. In: Experimental unsatured soil mechanics. Vol. 112. Part. I. 79-93. Schanz, T. (ed.), Springer, Berlin. [ Links ]
Chalmers Y.M., Krstic M.P., Downey M.O., Loveys B.R., Dry P.R., 2007. Physiological mechanism used by grapevine varieties to cope with water deficit. Acta Hort., 754, 495-499. [ Links ]
Chapman D.M., Roby G., Ebeler S.E., Guinard J.X., Matthews M.A., 2005. Sensory attributes of Cabernet Sauvignon wines made from vines with different water status. Aust. J. Grape Wine Res., 11, 339-347. [ Links ]
Cholpankulov E.D., Inchenkova O.P., Paredes P., Pereira L.S., 2008. Cotton irrigation scheduling in Central Asia: Model calibration and validation with consideration of groundwater contribution. Irrig. Drain., 57, 516-532. [ Links ]
Choné X., van Leeuwen C., Dubordieu D., Gaudillère J.P., 2001. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot., 87, 477-483. [ Links ]
Decagon Device, 2000. Operator´s manual WP4 Dewpoint PotentiaMeter. Decagon Devices, Inc., USA. [ Links ]
Deloire A., Carbonneau A., Wang Z., Ojeda H., 2004. Vine and water: a short review. J. Int. Sci. Vin., 38, 1-13. [ Links ]
Deloire A., Ferrer M., Carbonneau A., 2003. Respuestas de la viña al terroir. Elementos para un método de estudio. Agrociencia, VII, 105-113. [ Links ]
Díaz-Zorita M., Grove J.H., Murdock L., Herbeck J., Perfect E., 2004. Soil structural disturbance effects on crop yields and soil properties in a no-till production system. Agron. J., 96, 1651-1659. [ Links ]
Duniway J.M., 1971. Comparison of pressure chamber and the thermocouple psychrometer determinations of leaf water status in tomato. Plant Physiol., 48, 106-107. [ Links ]
Escalona J.M., Fuentes S., Tomás M., Martorell S., Flexas J., Medrano H., 2013. Responses of leaf night transpiration to drought stress in Vitis vinifera L. Agr. Water Manage., 118, 50-58. [ Links ]
Fandiño M., Cancela J.J., Rey B.J., Martínez E.M., Rosa R.G., Pereira L.S., 2012. Using the dual-Kc approach to model evapotranspiration of Albariño vineyards (Vitis vinifera L. cv. Albariño) with consideration of active ground cover. Agr. Water Manage., 112, 75-87. [ Links ]
Ferreyra R., Selles G., Maldonado P., Celedón A., Gil P., 2007. Efecto del clima, de las características de la hoja y de la metodología de medición en el potencial hídrico xilemático en Palto (Persea americana Mill.). Agric. Téc., 67, 182-188. [ Links ]
Ferreyra R., Selles G., Peralta J., Burgos L., Valenzuela J., 2002. Efectos de la restricción del riego en distintos periodos de desarrollo de la vid cv. Cabernet Sauvignon sobre producción y calidad del vino. Agric. Téc., 62, 406-417. [ Links ]
Ferreyra R., Selles G., Silva H., Ahumada R., Muñoz I., Muñoz V., 2006. Efecto del agua aplicada en las relaciones hídricas y productividad de la vid Crimson seedless. Pesq. Agropec. Bras. Brasília, 41, 1109-1118. [ Links ]
Fredlund D.G., Rahardjo H., Fredlund M.D., 2012. Unsatured soil mechanics in engineering practice. 926 p. John Wiley & Sons, USA. [ Links ]
Gee G.W., Campbell M.D., Campbell G.S., Campbell J.H., 1992. Rapid measurement of low soil water potentials using a water activity meter. Soil Sci. Soc. Am. J., 56, 1068-1070. [ Links ]
Ghaderi N., Siosemardeh A., Shahoei S., 2007. The effect of water stress on some physiological characteristics in `Rashe and `Khoshnave grape cultivars. Acta Hort., 754, 317-322. [ Links ]
Giorgessi F., Calò A., Sansone L., 1998. Importanza dellirrigazione per la qualità del prodotto ed influenza di alcune tecniche colturali sul fabbisogno idrico della cv. Cabernet sauvignon, nellambiente dellItalia nordorientale. Riv. Vitc. Enol. 4, 3-12. [ Links ]
Girona J., Mata M., del Campo J., Arbonés A., Bartra E., Marsal J., 2006. The use of midday leaf water potential for scheduling deficit irrigation in vineyards. Irrig. Sci., 24, 115-127. [ Links ]
Green S., Dryden G., Neal S., Davidson P., Martin D., Neal M., Clothier B., 2003. The effect of reduced irrigation on yield and quality of Sauvignon Blanc grapes in Marlborough. Grapegrower & Winemaker, 474, 101-104. [ Links ]
Hidalgo J., 2006. La calidad del vino desde el viñedo. 389 p. Ed. Mundi-Prensa, Madrid. [ Links ]
Hidalgo L., 1993. Tratado de viticultura general. 983 p. Ed. Mundi-Prensa, Madrid. [ Links ]
Intrigliolo D., Castel J.R., Chirivella C., 2007a. Response of grapevine cv. Tempranillo to irrigation amount and partial rootzone drying under contrasting crop load levels. Acta Hort., 754, 309-316. [ Links ]
Intrigliolo D., Ferrer P.J., Castel J.R., 2007b. Monitorización del riego en vid. In: Fundamentos, aplicación y consecuencias del riego en la vid. 83-113. Baeza P., Lissarrague J.R., Sánchez, P., (eds.), Ed. Agrícola, S.A., Madrid. [ Links ]
Itier B., Katerji N., Flura D., Ferreira I., 1990. Relative evapotranspiration in relation to soil water deficit and predawn leaf water potential. Application to tomato crop. Acta Hort., 278, 101-111. [ Links ]
Jackson D., Lombart P., 1993. Environmental and management practices affecting grape composition and wine quality. A review. Am. J. Enol. Vitic., 44, 409-430. [ Links ]
Johnson L.F., Herwitza S., Dunagana S., Lobitza B., Sullivan D., Slye R., 2003. Collection of ultra high spatial and spectral resolution image data over California vineyards with a small UAV. In: Proc 30th International Symposium on Remote Sensing of Environment. CD paper TS-12.4, Honolulu. [ Links ]
Katerji N., van Hoorn J.W., Hamdy A., Mastrorilli M., 2000. Salt tolerance classification of crops according to soil salinity and to water stress day index. Agric. Water Manage., 43, 99-109. [ Links ]
Kikuta S.B., Kyriakopoulous E., Richter H., 1985. Leaf hygrometer v. pressure chamber: a comparison of pressure-volume curve data obtained on single leaves by alternating measurements. Plant Cell Environ., 8, 363-367. [ Links ]
Kirkham M.B., 2005. Principles of soil and plant water relations. 500 p. Elsevier Academic Press, New York. [ Links ]
Klepper B., Barrs H.D., 1968. Effects of salt secretion on psychrometic determinations of water potential of cotton leaves. Plant Physiol., 43, 1138-1140. [ Links ]
Kondo S., Kittikorn M., Ohara H., Okawa K., Sugaya S., Kitahata N., Asami T., 2009. Effect of a cytochrome P450 inhibitor on abscisic acid biosynthesis and stomatal regulation in citrus trees in water-stressed conditions. Sci. Hort., 120, 146-149. [ Links ]
Lechaudel M., Vercambre G., Lescourret F., Normand F., Génard M., 2007. An analysis of elastic and plastic fruit growth of mango in response to various assimilate supplies. Tree Physiol., 27, 219-230. [ Links ]
Livingston N.J., Topp G.C., 2006. Soil water potential. In: Soil sampling and methods of analysis. 963-979. Carter M.R., Gregorich G., (eds.), Taylor and Francis Group, LLC. [ Links ]
Londers E., Ceusters J., Vervaeke I., Deroose R., de Proft M.P., 2005. Organic acid analysis and plant water status of two Aechmea cultivars grown under greenhouse conditions: implications on leaf quality. Sci. Hort., 105, 249-262. [ Links ]
Lopes C.M., Monteiro A., Machado J.P., Fernandes N., Araújo A., 2008. Cover cropping in a slopping non-irrigated vineyard: II - effects on vegetative growth, yield, berry and wine quality of cabernet sauvignon grapevines. Ciência Téc. Vitiv., 23, 37-43. [ Links ]
Maggio A., Raimondi G., Martino A., De Pascale S., 2007. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot., 59, 276-282. [ Links ]
Maringo G., Peltier J.P., 1996. Analysis of the diurnal change in osmotic potential in leaves of Fraxinus excelsior L. J. Exp. Bot., 47, 763-769. [ Links ]
Martínez E.M., 2008. Estudio de propiedades hídricas del suelo mediante medidores de actividad de agua en la zona regable de Terra Chá. 496 p. PhD Thesis, Universidad de Santiago de Compostela. [ Links ]
Martínez E.M., Cancela J.J., 2009. Calibración de un medidor de actividad de agua: Punto de marchitamiento permanente. In: V Congreso Nacional y II Congreso Ibérico AgroIngeniería. 237-238. Dpto. Ing. Agroforestal (ed.), UNICOPIA, Lugo. [ Links ]
Martínez E.M., Cancela J.J., 2011. Condiciones de contorno en las determinaciones del punto de marchitamiento permanente con water activity meters. Span. J. Rural Dev., 2, 1-14. [ Links ]
Martínez E.M., Cancela J.J., Cuesta T.S., Neira X.X., 2007. Efecto de la temperatura en el uso de un medidor de la actividad de agua. In: XXV Congreso Nacional de Riegos. 29-31. Gobierno de Navarra (ed.), ONA S.A., Pamplona. [ Links ]
Martínez E.M., Cancela J.J., Cuesta T.S., Neira X.X., 2011. Review. Use of psychrometers in field measurements of plant material: accuracy and handling difficulties. Span. J. Agric. Res., 9, 313-328. [ Links ]
McBurney T., Costigan P.A., 1987. Plant water potential measured continuously in the field. Plant Soil, 97, 145-149. [ Links ]
Medrano H., Escalona J.M., Flexas J., 2007. Indicadores integradores del estado hídrico de la planta. In: Fundamentos, aplicación y consecuencias del riego en la vid. 15-34. Baeza P., Lissarrague J.R., Sanchez P. (eds.), Ed. Agrícola Española S.A., Madrid. [ Links ]
Millar B.D., Hansen G.K., 1975. Exclusion errors in pressure chamber estimates of leaf water potential. Ann. Bot., 39, 915-920. [ Links ]
Mullins C.E., 2001. Matric potential. In: Soil and environmental analysis. Physical methods. 65-93. Smith K.A., Mullins C.E., (eds.), Marcel Dekker Inc, New York. [ Links ]
Murray E.J., Sivakumar V., 2010. Unsatured soils: A fundamental interpretation of soil behavior. 304 p. Wiley-Blackwell, UK. [ Links ]
Nadal M., Arola L., 1995. Effects of limited irrigation on the composition of must and wine of Cabernet Sauvignon under semi-arid conditions. Vitis, 34, 151-154. [ Links ]
Nadal M., Lampreave M., 2004. Influence de lirrigation sur la résponse hydrique du cep, le rendement et la composition des vins du cv Tempranillo cv. in Mediterranean climate. J. Int. Sci. Vigne Vin, 38, 75-80. [ Links ]
Naor A., Bravdo B., Hepner Y., 1993. Effect of post-veraison irrigation level on Sauvignon blanc yield, juice quality and water relations. S. Afr. J. Enol. Vitic., 14, 19-25. [ Links ]
O´Neill S.D., 1983. Role of osmotic potential gradients during water stress and leaf senescence in Fragaria virginiana. Plant Physiol., 72, 931-937. [ Links ]
Padilla F.M., Miranda J.D., Jorquera M.J., Pugnaire F.I., 2009. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol., 204, 261-270. [ Links ]
Patel A.D., Pandey A.N., 2007. Effect of soil salinity on growth, water status and nutrient accumulation in seedlings of Cassia montana (Fabaceae). J. Arid Environ., 70, 174-182. [ Links ]
Peel M.C., Finlayson B.L. McMahon T.A., 2007. Updated world map of the Köppen-Geiger climate classification, Hydrol. Earth Syst. Sci., 11, 1633-1644. [ Links ]
Petrie P.R., Cooley N.M., Clingeleffer P.R., 2004. The effects of post-veraison water deficit on yield components and maturation of irrigated Shiraz (Vitis vinifera L.) in the current and following season. Aust J. Grape Wine Res., 10, 203-215. [ Links ]
Petry T.M., Jiang C.P., 2003 Evaluation and utilization of the WP4 dewpoint potentiameter Phase I and II. Center for Infrastructure Engineering Studies. University Transportation Center Program, University of Missouri-Rolla, Missouri. [ Links ]
Phillips D.L., 1981. End-point recognition in pressure chamber measurements of water potential of Viguiera porter (Asteraceae). Ann. Bot., 48, 905-907. [ Links ]
Pire R., de Pire M.L., Tortolero E., de Fréitez Y., 1988. El riego de la vid (Vitis vinifera L.) en el Toyuco. Estado Lara. II. Relaciones suelo agua. Agronomía Tropical, 38, 155-171. [ Links ]
Richter H., 1976. The water status in the plant experimental evidence. In: Water and plant life. Vol. 19. 42-58. Lange O.L., Kappen L., Schulze E.-D. (ed.). Springer Berlin Heidelberg, Berlin. [ Links ]
Ritchie G.A., Hinckley T.M., 1975. The pressure chamber as an instrument for ecological research. Adc. Ecol. Res., 9, 165-254. [ Links ]
Rivero R.M., Kojima M., Gepstein A., Sakakibara H., Mittler R., Gepstein S., Blumwald E., 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. PANS., 104, 19631-19636. [ Links ]
Roby G., Harbertson J.F., Adams D.A., Matthews M.A., 2004. Berry size and wine water deficits as factor in winegrape composition: anthocyanins and tannins. Aust. J. Grape Wine Res., 10, 100-107. [ Links ]
Santalucia G., Barbagallo M.G., Costaza P., Lorenzo D., Pisciotta A., 2007. Vegetative and reproductive behaviour of Vitis vinifera L. (cv. Cabernet Sauvignon) vines growing under non-irrigated conditions and moderate water stress induced by different irrigation systems. Acta Hort., 754, 323-328. [ Links ]
Santesteban L.G., Miranda C., Royo J.B., 2007. Regulated deficit irrigation effects in cv. `Tempranillo´ vineyards grown under semiarid conditions in Mid-Ebro river valley (Spain). Acta Hort., 754, 501-506. [ Links ]
Scanlon B.R., Andraski B.J., Bilskie J., 2002. Miscellaneous methods for measuring matric or water potential. In: Methods of soil analysis-Part 4: Physical Methods. 643-670. Dane J.H., Topp G.C. (eds.), SSSA, Madison. [ Links ]
Scholander P.F., Hammel H.J., Bradstreet A., Hwemmingsen E.A., 1965. Sap pressure in vascular plants. Science, 148, 339-346. [ Links ]
Smart R.E., Coombe B.G., 1983. Water relations of grapevines. In: Water deficit and plant growth. Vol. 7. 137-196. Kozlowski T.T. (ed.), Academic press, New York. [ Links ]
Tardieu F., Katerji N., Bethenod O., Zhang J., Davies W.J., 1991. Maize stomatal conductance in the field: its relationship with soil and plant water potentials, mechanical constraints and ABA concentration in the xylem sap. Plant Cell Environ., 14, 121-126. [ Links ]
Thakur V.K.S., Sreedeep S., Singh D.N., 2006. Laboratory investigations on extremely high suction measurements for fine-grained soils. Geotech. Geol. Eng., 24, 565-578. [ Links ]
Turner N.C., 1981. Correction of flow resistances of plants measured from covered and exposed leaves. Plant Physiol., 68, 1090-1092. [ Links ]
Turner N.C., Long M.J., 1980. Errors arising from rapid water loss in the measurement of leaf water potential by the pressure chamber technique. Aust. J. Plant Physiol., 7, 527-537. [ Links ]
Turner N.C., Shackel K.A., Le Coultre I.F., 2000. Leaf-cutter psychrometers. Agron. J., 92, 538-541. [ Links ]
Van Leeuwen C., Pieri P., Vivin P., 2010. Comparison of three operational tools for the assessment of vine water status: stem water potential, carbon isotope discrimination measured on grape sugar and water balance. In: Methodologies and results in grapevine research. 87-106. Delrot S., Medrano H., Or E., Bavaresco L., Grando S., (eds.). Springer, Netherlands. [ Links ]
Van Leeuwen C., Tregoat O., Choné X., Bois B., Pernet D., Gaudillère J.P., 2009. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux vine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin, 43, 121-134. [ Links ]
Van Zyl J.L., 1987. Diurnal variation in grapevine water stress as a function of changing soil water status and meteorological conditions. S. Afr. J. Enol. Vitic., 8, 45-52. [ Links ]
Walker R.R., Blackmore D.H., Clingeleffer P.R., Correll R.L., 2002. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. Sultana). 1. Yield and vigour inter-relationships. Aust. J. Grape Wine Res., 8, 3-14. [ Links ]
Williams L.E., Araujo F.J., 2002. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J. Am. Soc. Hort. Sci., 127, 448-454. [ Links ]
Xu Z., Zhou G., 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot., 59, 3317-3325. [ Links ]
Yuste J., Gutiérrez I., Rubio J.A., Alburquerque M.V., 2004. Résponse des potentiels hydriques de la feuille et du xylème comme indidateurs de létat hydrique de la vigne, cépage tempranillo, soumis à différents régimes hydriques dans la vallée du Douro. J. Int. Sci. Vigne Vin., 38, 21-26.
Zufferey V., 2007. Alimentation en eau et irrigation de la vigne. Rev. S. Vitic. Arboric. Hortic., 39, 77-78. [ Links ]
*Corresponding author:
Tel:+34 678920897 e-mail: javierjose.cancela@usc.es
(Manuscrito recebido em 20.05.2013. Aceite para publicação em 24.10.2013)













