Serviços Personalizados
Journal
Artigo
Indicadores
Links relacionados
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. vol.19 n.Especial Lisboa 2011
Stone Pine Volatiles and Host Selection by Tomicus destruens (Wollaston) (Coleoptera: Curculionidae, Scolytidae)
1 Massimo Faccoli, 2 Gianfranco Anfora and 2 Marco Tasin
1 Department of Environmental Agronomy and Crop Sciences – Entomology Agripolis – Viale dell'Università, 16/a – 350201 Legnaro (PD). ITALY
2 Plant Protection Department and Safe Crop Centre. IASMA Research Center. Via E. Mach I – 38010 San Michele all'Adige. ITALY
Abstract
Mature adults of the pine shoot beetle Tomicus destruens reproduce in trunks of dying Mediterranean pines. However, there is no information about chemical stimuli governing host selection by T. destruens. The aims of this study were: 1) to identify volatiles released by the bark of stone pines (Pinus pinea) behaviorally and electro-physiologically active on T. destruens; 2) to verify which blends and concentrations of such volatiles act differently on males and females. A four-arm olfactometer was used to test behavior of walking adults of T. destruens in relation to various sources of volatiles, including bark, its collected volatiles and a synthetic blend. For each odor, the behavior of both males and females was recorded individually. Bark extracts were then analyzed by coupled gas chromatography and mass spectrometry (GC–MS) and tested by gas-chromatography coupled with electro-antennography (GC-EAD) on male and female antennae of T. destruens. In addition, a blend of synthetic compounds chosen from among those inducing EAD responseswas tested in the olfactometer at five concentrations. Behavior and EAD responses were not affected by sex, with males and females showing similar results. Five compounds extracted from bark were active on T. destruens antennae: limonene, β-caryophyllene, α-pinene, β-myrcene, and α-terpinolene. Among extracted volatiles, limonene and β-caryophyllene were known to be repellent to T. destruens, whereas attraction to a synthetic blend of α-pinene, β-myrcene and α-terpinolene was positively correlated with concentration, although a repellent effect was noted at the highest concentrations.
Key words: Host selection; kairomones; electrophysiology; olfactometry; terpenes; Pinus; Blastophagus
Voláteis de Pinheiro-Manso e Seleção do Hospedeiro pela Hilésina Tomicus destruens (Wollaston) (Coleoptera: Curculionidae, Scolytidae)
Sumário
Os adultos da hilésina Tomicus destruens reproduzem-se em troncos de várias espécies de pinheiros da região mediterrânica. No entanto, não se conhecem os estímulos químicos que condicionam a respetiva seleção dos hospedeiros. Os objetivos do presente estudo são: 1) identificar os voláteis da casca do pinheiro-manso (Pinus pinea) que influenciam a resposta eletrofisiológica e comportamental de T. destruens e 2) determinar quais as misturas e concentrações de odores que afetam diferencialmente os dois sexos. A atratividade dos adultos da hilésina em relação aos voláteis (incluindo casca, voláteis da casca e uma mistura sintética) foi estudada usando um olfatómetro de quatro braços. Para cada odor o comportamento de machos e fêmeas foi estudado individualmente. Amostras de casca foram analisadas através de cromatografia gasosa e espectrometria de massa (GC–MS), tendo sido testados em machos e fêmeas de T. destruens através de cromatografia gasosa acoplada a eletroantenografia (GC-EAD). Adicionalmente, uma mistura sintética de compostos escolhidos entre aqueles que induziram respostas na EAD foi testada no olfatómetro, com cinco concentrações distintas. Constatou-se que o comportamento e a resposta eletroantenográfica dos insetos não dependiam do sexo; tanto machos como fêmeas deram resultados semelhantes. Cinco compostos extraídos da casca dos pinheiros desencadearam respostas ativas nas antenas de T. destruens, nomeadamente: limoneno, β-cariofileno α-pineno, β-mirceno e α-terpinoleno. Destes, limoneno e β-cariofileno foram identificados como repelentes para a hilésina, enquanto que, para a mistura sintética de α-pineno, β-myrceno e α-terpinoleno, foi detetada uma correlação positiva entre a atratividade e a concentração, embora a concentrações mais altas tenha ocorrido um efeito repelente.
Palavras-chave: Seleção de hospedeiro; cairomonas; eletrofisiologia; olfatometria; terpenos; Pinus, Blastophagus
Introduction
Host selection by bark beetles is mainly governed by volatile attractive signals (WOOD, 1982). In many cases, individuals of only one sex (pioneers) find suitable hosts and begin to attack; then they release a species-specific aggregation pheromone (BYERS, 1989). Later, specimens of the non-host-searching sex join the pioneers, simply by orienting to the pheromone. The pine shoot beetle Tomicus destruens (WOLLASTON, 1865) (Coleoptera, Curculionidae Scolytidae) is among the most damaging pests of Mediterranean pine forests (CHARARAS, 1962; FACCOLI et al., 2005a; FACCOLI, 2007; VASCONCELOS et al., 2005). Similarly to other monogamous species, females are the pioneer sex, infesting the host tree first, whereas males arrive on the bark later. Although a secondary attraction of sexual origin has been reported in T. destruens (CARLE, 1974; 1978; CARLE et al., 1978), the effective aggregation pheromone has never been found, and adult aggregation appears to be mediated only by host volatiles (GUERRERO et al., 1997). In some cases, efforts to trap T. destruens have been made with commercially available lures for T. piniperda, a closely related species living mainly on Scots pine (P. sylvestris) and often considered synonymous with T. destruens until their recent systematic revision (FACCOLI et al., 2005b; FACCOLI, 2006). The bait contains a synergistic blend of the host volatile a-pinene and ethanol (CZOKAJLO and TEALE, 1999), with or without added 3-carene and a-terpinolene (BYERS et al., 1985; SCHRÖEDER and EIDMANN, 1987; POLAND et al., 2004). One of the main monoterpenes released by conifers is a-pinene and ethanol is a product of the anaerobic fermentation of phloematic sugars in weakened and dying trees (KIMMERER and KOZLOWSKI, 1982). However, the number of adult T. destruens captured in traps baited only with a-pinene and ethanol is always very low (SABBATINI PEVERIERI et al., 2004; VASCONCELOS et al., 2005) when compared with catches of other bark beetles. In this respect, adult T. destruens looking for hosts susceptible for colonization probably follow chemical cues more specific than a-pinene and ethanol; otherwise the beetle would infest any conifer having physiological problems. Moreover, the hosts are frequently present in mixed stands together with non-host species and flying adults need effective cues to find suitable breeding material (GUERRERO et al., 1997).
The aims of the present study were: 1) to identify the volatiles released by the bark of stone pines (Pinus pinea) that are behaviorally and electro-physiologically active on T. destruens and 2) to verify which blends and concentrations of such volatiles act differently on males and females during host search.
Materials and methods
Sample collection and handling
Adults of T. destruens were collected during the winter in 2004 from a stone pine forest in NE Italy (Caorle (VE), 45°54'N; 12°36'E). They were extracted from pine shoots recently fallen on the litter following attack. The insects were then identified and sexed individually by acoustical and morphological features (Faccoli, 2006). Logs of healthy pines used for chemical and behavioral studies were collected in the same stand where the insects were sampled.
Odor collection
Headspace collections were made from fresh bark of stone pine. A freshly cut log (ca. 800 g, length 20 cm, diameter 10 cm) was placed into a 2000-ml glass jar. The cut ends of the log were sealed with paraffin. Charcoal-filtered air was pumped into the jar at 150 ml/min, over a Porapak Q cartridge containing 50 mg of adsorbent (Sigma-Aldrich, Milan, Italy). Collection was made for 24 hr in a climatic chamber at 25 ± 2 ºC, 60 ± 10% R.H., 16L: 8D photoperiod and 1000 lux during the light period (TASIN et al., 2005). Volatiles were desorbed by eluting the cartridge with 500 µl of redistilled hexane. For quantification, additional extracts (N = 5) of bark were prepared, and 0.5 µg of heptyl acetate (purity ³ 99%) were added as an internal standard (BENGTSSON et al., 2001). Sample volumes were reduced to 50 μl at room temperature. Samples were sealed in glass vials and stored at −18°C.
Chemical analysis
Five samples of the extract were analyzed by coupled gas chromatography and mass spectrometry (GC–MS). Analyses were performed on a Hewlett-Packard 5890 GC, with a polar Innowax column (30 m x 0.32 mm; J & W Scientific, Folsom, CA) programmed from 60°C (hold 3 min) at 8°C min-1 to 220°C (hold 7 min), interfaced with a Hewlett-Packard 5970B MS with electron impact ionization (70 eV). The identity of compounds in volatile collections was verified by comparison with synthetic compounds purchased from Sigma-Aldrich and Fluka Chemie. Compounds that did not elicit antennal responses and for which no standards were available were tentatively identified using the Wiley mass spectral database. Identified compounds were quantified by comparing their peak areas with those of the internal standard (ZHANG et al., 2007).
Gas chromatography and electro-antennographic detection (GC-EAD)
Two microliters of the extract were injected into a Hewlett-Packard 5890 GC, with a polar Innowax column (30 m x 0.32 mm; J & W Scientific, Folsom, CA) programmed from 60°C (hold 3 min) at 8°C min-1 to 220°C (hold 7 min), interfaced with an electroantennogram apparatus (ARN et al., 1975). The outlet of the GC column was split in a 1:1 ratio between the flame ionization detector (FID) and one of the antennae of T. destruens. A glass capillary indifferent electrode filled with Kaissling solution with an added 5 g l-1 polyvinylpyr-rolidone K90 (Fluka Chemie) was inserted into the severed head of the beetle; the different electrode was a similar pipette, brought into contact with the distal end of the antennal club. Compounds eluting from the capillary column were delivered to the antenna through a glass tube (12 cm × 8 mm) by a charcoal-filtered and humidified airstream. The antennal and FID signals were amplified and recorded simultaneously by SyntechÒ software (Hilversum, The Netherlands). Samples from bark extracts were tested on five males and five females of T. destruens. A compound was considered electro-physiologically active when it elicited at least three antennal responses different from background noise (ZHANG et al., 2001).
Behavioral responses
A four-arm olfactometer, in which individual insects could walk freely (VET et al.,1983), was used to test the behavioral responses of walking adults of T. destruens to various sources of volatiles. Incoming air (about 800 ml/minute) passed through an activated carbon filter (Whatman Carbon-Cap 75) before being branched off to separate but identical lines leading to the olfactometer corners, each connected to a 300 ml glass jar containing the odor source. Finally, exiting airflows were conveyed into the center of the olfactometer floor, where a vial contained a test insect (VET et al., 1983). Only one arm of the olfactometer contained a test odor (called Arm 1), whereas the other arms were empty. A digital camera, positioned centrally above the chambers, was linked to a video recording monitor. Digital records were analyzed later, as described below.
The selected sources of volatiles included fresh bark, its collected volatiles, a blend of synthetic compounds and a blank (an empty olfactometer). For each source and concentration, 10 adults were tested, taking sex into account (male/female). Fresh material - randomly collected from several trees -was tested, with 50 g of bark inserted directly into a jar connected with arm 1; bark extracts were tested with 10 μl per insect. Finally, a blend of three synthetic compounds (α-pinene, β-myrcene and α-terpinolene) chosen among those inducing GC-EAD responses and known to be attractive to other bark beetle species (see results) were tested at five concentrations ranging from 10-2 to 102 of the amounts collected from 800 g of a cut branch (Table 1). The blend was prepared by following the proportions of α-pinene, β-myrcene and α-terpinolene occurring in the plume collected from bark (18: 80: 2). For every five insects tested, the whole apparatus was washed and Arm 1 randomly reassigned. Experiments were carried out in laboratory conditions (21±1°C, 70% R.H.), assuming a similar concentration of volatiles, released by samples kept at the same temperature and having the same size and similar physiological conditions. The behavior of T. destruens adults was recorded individually for 10 minutes; each insect and each sample tissue was only used once.
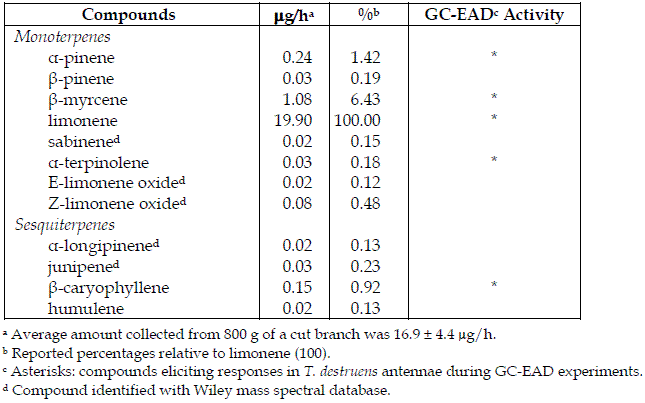
Table 1 - Volatile compounds collected in headspace of Pinus pinea bark and antennal activity in GC-EAD experiments on Tomicus destruens adults
Analysis of behavioral data
The digital record of the activity of each insect in the olfactometer was analyzed by the Micro Measure® program (Wye College Programs). A video mixing desk was used to lay out a computer-generated map of the olfactometer on the video image. The borders of the odor fields were drawn on the computer image and labeled as zones. All the flying or walking tracks of the insect were traced from the video image by a cursor. For each insect tested and for each arm of the olfactometer, the program measured time spent walking (sec), time spent stationary (sec), and total time spent (sec) in each arm. Data were analyzed by multi-way ANOVA to find statistical differences in time spent by adults in Arm 1, in relation to their sex and tested odor. Possible interactions among the investigated variables were also analyzed. Homogeneity of variance was tested by Cochran's test (test C) and normality by Kolmogorov-Smirnow's test (test D); when necessary, data were transformed by log (X' = log (x+1)) or arcsine (X' = arcsineÖx) to obtain homogeneous data and normal variance. Whenever significant differences occurred, Tukey's honestly significant difference (HSD) multiple comparison test was applied for mean separation (ZAR, 1999). Differences at the 0.05 level of confidence, adjusted by Bonferroni correction for mass comparison, were considered significant. Analyses were performed by STATISTICA® 3.1 for WINDOWS® software (Statistica®, Tulsa, OK).
Results
Chemical analysis
Compounds identified from the bark of stone pine (Table 1) only included eight monoterpenes and four sesquiterpenes. The most abundant compound in headspace collections was limonene, with an average amount - from 800 g of a cut log - of 16.9 ± 4.4 µg/h (ca. 91% of the whole blend), followed by β-myrcene (6.4%), α-pinene (1.4%) and β-caryophyllene (0.92%) (Table 1).
Gas chromatography and electro-antennographic detection (GC-EAD)
GC-EAD analyses of headspace collections detected five active compounds on T. destruens antennae (Table 1). Considering antennal responses and the quantity of compounds in the blend, monoterpenes α-pinene, β-myrcene and α-terpinolene were the most antennally active volatiles (Figure 1), and were therefore later tested in the olfactometer (see "methods and materials"). Antennal responses were also elicited by limonene and β-caryophyllene (Figure 1 and Table 1). Both males and females showed similar EAD responses.
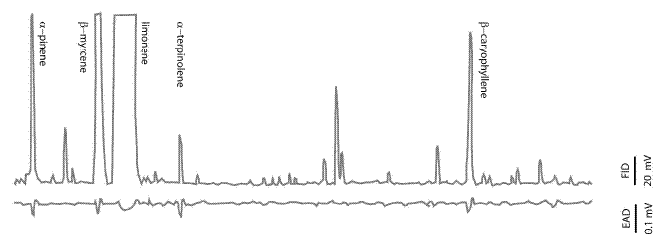
Figure 1 - GC-EAD responses of Tomicus destruens to headspace collection from bark of stone pine. Compounds eliciting antennal responses: α-pinene, β-myrcene, limonene, α-terpinolene, and β-caryophyllene (see Table 1)
Behavioral responses
When exposed to the same source, males and females showed similar behavior (F1, 58 = 1.32, P = 0.12) (Figure 2). Thus, further analyses were performed with joined male and female data. Adults were clearly attracted by bark and its extracts, with statistical differences from the blank (F2, 57 = 7.13, P < 0.01) (Figure 2). The responses of adults to the synthetic blend were correlated with the blend concentration by a logarithmic equation (Figure 3), with responses becoming significantly higher than the blank at blend concentrations exceeding or equal to 1 (F5, 58 = 3.46, P < 0.001) (Figure 3).
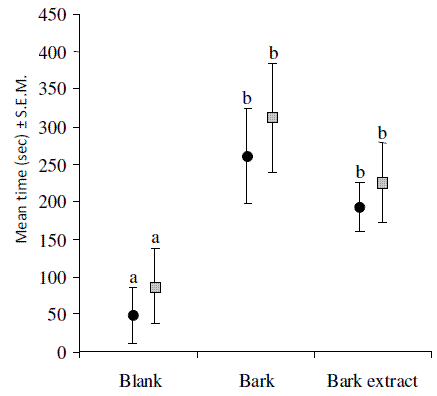
Figure 2 - Mean time spent by males and females of Tomicus destruens in the olfactometer arm containing the tested odors (arm 1). Black dots: females; gray squares: males. Blank means empty olfactometer. Differences analyzed by two-way ANOVA on odors and sexes. Different letters mean statistical differences by Tukey test (P<0.05)
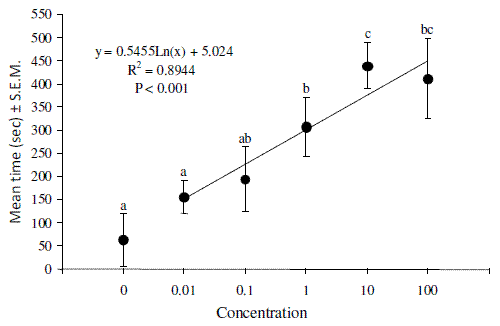
Figure 3 - Mean time spent by adults of Tomicus destruens in the olfactometer arm containing the tested concentrations of the synthetic blend (α-pinene, β-myrcene and α-terpinolene). Concentration equal to 0 is the blank (empty olfactometer), whereas concentration equal to 1 is the amount of volatiles collected from 800 g of a cut branch (Table 1). Different letters among concentrations mean statistical differences by Tukey test (P<0.05)
Discussion
Our results indicate that the behavior of T. destruens is governed by specific volatile cues. In this respect, stopping or slowing walking speed is usually considered to be a response to a chemical trace of an attractive host (JACOBSON, 1966). Although it has been demonstrated that the behavior of many insects may be affected by odors encountered during previous searches, each adult was tested only once, to avoid any possible effect on responses resulting from previous exposure. Also, the intensity of the odor to which an insect is exposed can affect its activity, and several species fail to respond to low or high concentrations of a kairomone (JONES et al., 1971). In the synthetic blend tested in the present study, concentrations equal to or higher than those naturally released by bark and shoots caused significant alterations in behavior compared with clean air (empty arms), indicating that the odor flow was strong enough to elicit the insect's responses. Adult response increased with concentration, although a blend concentration a hundred times higher than the natural plume seemed to cause repellence, as observed in other bark beetle species (FACCOLI et al., 2005c).
In the present study, gender did not affect the behavioral or EAD responses to host volatiles. When exposed to host material or host extracts, males and females showed similar behavior, and both sexes had similar EAD responses to all tested volatiles. Higher antennal sensitivity (lower response threshold) would be expected for the host-selecting sex (pioneers) - females for monogamous genera such as Scolytus and Tomicus, and males for polygamous ones, like Ips and Pityogenes. For instance, previous studies report the pioneer sex of I. typographus as more affected by host and non-host volatiles (FACCOLI et al., 2005c). DICKENS (1981) found that females of I. typographus, which find suitable hosts following male-produced pheromones, do not have high sensitivity to host volatiles, and that their antennae have fewer receptors responsive to α-pinene than those of males. When responding to pre-landing signals, males of I. typographus are more sensitive than females to verbenone (SCHLYTER et al., 1989) and non-host volatiles (ZHANG and SCHLYTER, 2004), whereas females of Scolytus rugulosus are more susceptible to anti-feedants than males (ASCHER et al., 1975). In addition, BYERS et al. (1998, 2004) found that males of the polygamous P. chalcographus and P. bidentatus avoid non-host trees more than females. However, this mechanism seems to be different in Tomicus species. Adults of T. piniperda are attracted to pine logs or to bark monoterpenes in an equal sex ratio (LÅNGSTRÖM, 1980; 1983; BYERS et al., 1985; LANNE et al., 1987) and our results also confirm this finding for T. destruens.
No aggregation pheromone has been found for T. destruens; T. piniperda, a more frequently studied species, has also given unsatisfactory results. Laboratory bioassays with walking beetles and field tests have shown that T. piniperda is equally attracted to infested and uninfested pine logs (BYERS et al., 1985; LANNE et al., 1987). Similarly, PERTTUNEN et al. (1970) found that the presence of boring females did not enhance log attractiveness, indicating that, in T. piniperda, host and mate location are achieved only by host volatiles. In this respect, several studies have demonstrated that conifer phloem contains extractable compounds attractive to bark beetles (BYERS, 1995). In our experiments, the only compounds eliciting antennal responses in T. destruens were four monoterpenes (α-pinene, β-myrcene, α-terpinolene and limonene) and one sesquiterpene (β-caryophyllene). These volatiles are very common, and their attractive or repellent effect on bark beetles is well known. For instance, field investigations report b-caryophyllene as a repellent, reducing the attraction of P. bidentatus to pheromone-baited traps (BYERS et al., 2004). High contents of b-caryophyllene were also found in samples of oak twigs attacked by S. intricatus during maturation feeding and in oak logs during the boring of maternal galleries (VRKOCOVA et al., 2000), perhaps due to a reaction by the tree to tissue infestation by bark beetles. Limonene was the major volatile compound isolated from bark and shoots of P. pinea, as found by MACCHIONI et al. (2003). Limonene is one of the host monoterpenes involved in pre-existing and induced defenses against bark beetles (BAIER et al.,1999) and high concentrations occur in trees responding to an attack (ERBILGIN and RAFFA, 2000). Many experiments suggest that high concentrations of limonene are toxic or repellent to T. destruens (SABBATINI PEVERIERI et al., 2004) and other species, such as Dendroctonus brevicomis (STURGEON, 1979), I. pini (WALLIN and RAFFA, 2000), and I. typographus (EVERAERTS et al., 1988). However, much of the previous literature on monoterpene toxicity had methodological problems, since the concentrations necessary to kill beetles were much higher than in galleries under bark (BYERS, 1981).
Differently, a-pinene, a-terpinolene and b-myrcene are the only volatiles detected from P. pinea that are attractive to T. destruens. The role of a-pinene as a kairomone of T. piniperda (SCHRÖEDER and EIDMANN, 1987) and more generally of many conifer-inhabiting bark beetles is well known (BYERS, 1995). Our results, together with earlier field investigations (SABBATINI PEVERIERI et al., 2004; VASCONCELOS et al., 2005), indicate that a-pinene is also active on T. destruens. Similar results concern a-terpinolene, which elicited EAG responses in T. destruens and has also been reported as an attractant for T. piniperda (BYERS et al., 1985; SCHRÖEDER and EIDMANN, 1987; POLAND et al., 2004). Lastly, b-myrcene induces clear antennal responses in T. destruens, suggesting its major role in host selection. b-myrcene is one of the most common components of oleoresins of Mediterranean pines (MACCHIONI et al., 2003) but occurs in very small amounts in continental pine species (IDZOJTIC et al., 2005). This may explain the preference of T. destruens for the Mediterranean pines and T. piniperda for the continental ones. In addition, b-myrcene is not attractive to T. piniperda (BYERS et al., 1985). Although recent papers report positive responses of some Chinese populations of T. piniperda to b-myrcene released by P. yunnanensis, a pine species growing in Yunnan (southern China) (BORG-KARLSON et al., 1999; ZHAO et al., 2002), T. piniperda from these populations was later ascribed to a new species (Tomicus yunnanensis Kirkendall & Faccoli) (KIRKENDALL et al., 2008).
In conclusion, in our laboratory bioassay the blend composed of a-pinene, a-terpinolene and b-myrcene acts as a kairomone on both males and females of T. destruens. Intuitively, each volatile could encode specific information for finding a suitable host, since a-pinene is the main conifer monoterpene, a-terpinolene occurs mainly in pines, and b-myrcene is typical of Mediterranean pines. This attractive blend may be improved by ethanol - as observed in T. piniperda (SCHRÖEDER and LINDELÖW, 1989) - released by weakened and dying trees. Because an effective lure can potentially protect Mediterranean pine forests from bark beetle attacks, further research concerning odor signals mediating host location is of interest in the management of T. destruens infestations.
References
ARN, H., STÄDLER, E., RAUSCHER, S., 1975. The electroantennographic detector – a selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z. Naturforsch 30: 722–725. [ Links ]
ASCHER, K.R.S., GUREVITZ, E., RENNEH, S., NEMNY, N.E., 1975. The penetration of females of the fruit bark beetle Scolytus mediterraneus Eggers into antifeedant-treated twigs in laboratory tests. Zeit. Pflanz. Pflanz. 82: 378–383. [ Links ]
BAIER, P., BADER, R., ROSNER, S., 1999. Monoterpene content and monoterpene emission of Norway spruce (Picea abies (L.) Karst.) bark in relation to primary attraction of bark beetles (Col., Scolytidae), pp. 249–259, in F. Lieutier, W.J. Mattson & M.R. Wagner (eds.). Physiology and Genetics of Tree-Phytophagous Interactions. INRA Editions, Versailles. [ Links ]
BENGTSSON, M., BÄCKMAN, A.-C., LIBLIKAS, I., RAMIREZ, M.I., BORG-KARLSON, A.K., ANSEBO, L., ANDERSON, P., LÖFQVIST, J., WITZGALL, P., 2001. Plant odor analysis of apple: antennal response of codling moth females to apple volatiles during phenological development. J. Agric. Food Chem. 49: 3736–3741. [ Links ]
BORG-KARLSON, A.K., PERSSON, M., CHRISTIANSSON, A., FÄLDT, J., LÅNGSTRÖM, B., LI, L., LIU, H., ZHOU, N., LIEUTIER, F., 1999. Relative amounts and enantiomeric compositions of monoterpene hydrocarbons in Pinusyunnanensis and Pinussylvestris, pp. 101–110, in F. lieutier, W.J. Mattson & M.R. Wagner (eds.). Physiology and Genetics of Tree-Phytophage Interactions. INRA Editions, Versailles.
BYERS, J.A., 1981. Pheromone biosynthesis in the bark beetle, Ips paraconfusus, during feeding or exposure to vapours of host plant precursors. Insect Biochem. 11: 563–569. [ Links ]
BYERS, J.A., 1989. Chemical ecology of bark beetles. Experientia 45: 271–283. [ Links ]
BYERS, J.A., 1995. Host-tree chemistry affecting colonization in bark beetles, pp. 154–193, in R.T. Cardè & W.J. Bell (eds.). Chemical Ecology of Insects, 2. Chapman & Hall, New York. [ Links ]
BYERS, J.A., LANNE, B.S., LÖFQVIST, J., SCHLYTER, F., BERGSTRÖM, G., 1985. Olfactory recognition of host-tree susceptibility by pine shoot beetles. Naturwissenschaften 72: 324–326. [ Links ]
BYERS, J.A., ZHANG, Q.-H., SCHLYTER, F., BIRGERSSON, G., 1998. Volatiles from nonhost birch trees inhibit pheromone response in spruce bark beetles. Naturwissenschaften 85: 557–561. [ Links ]
BYERS, J.A., ZHANG Q.-H., BIRGERSSON, G., 2004. Avoidance of nonhost plants by a bark beetle, Pityogenes bidentatus, in a forest of odors. Naturwissenschaften 91: 215–219. [ Links ]
CARLE, P., 1974. Mise en évidence d'une attraction secondaire d'origine sexuelle chez Blastophagus destruens Woll. (Col. Scolytidae). Ann. Zool. et Écol. Anim. 6: 539–550. [ Links ]
CARLE, P., 1978. Essais d'attraction en laboratoire et en forêt de Blastophagus (piniperda L. et destruens Woll.). Colloques de l'INRApp. 92–101. [ Links ]
CARLE, P., DESCOINS, C., GALLOIS, M., 1978. Phéromones des Blastophagus (piniperda L. et destruens Woll.). Colloques de l'INRA pp. 87–91. [ Links ]
CHARARAS, C., 1962. Scolytides des Conifères. Encyclopédie Entomologique 37. Lechevalier, Paris. [ Links ]
CZOKAJLO, D., TEALE, S.A., 1999. Synergistic effect of ethanol to a-pinene in primary attraction of the larger pine shoot beetle, Tomicus piniperda. J. Chem. Ecol. 25: 1121–1130. [ Links ]
DICKENS, J.C., 1981. Behavioral and electrophysiological responses of the bark beetle Ips typographus to potential pheromone components. Phys. Entomol. 6: 251–261. [ Links ]
ERBILGIN, N., RAFFA, K.F., 2000. Opposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. J. Chem. Ecol. 26: 2527–2548. [ Links ]
EVERAERTS, C., GRÉGOIRE, J.-C., MERLIN, J., 1988. The toxicity of Norway spruce monoterpenes to two bark beetle species and their associated, pp. 335–344, in W.J. Mattson, Levieux J. & Dagan C.B. (eds.). Mechanisms of Woody Plant Defenses Against Insects – Search for Pattern. Springer Verlag, New York. [ Links ]
FACCOLI, M., 2006. Morphological separation of Tomicus piniperda and T. destruens (Coleoptera, Curculionidae, Scolytinae): new and old characters. Eur. J. Entomol. 103: 433–442. [ Links ]
FACCOLI, M., 2007. Breeding performance and longevity of Tomicus destruens on Mediterranean and continental pine species. Ent. Exp. Appl. 123: 266–269. [ Links ]
FACCOLI, M., BATTISTI, A., MASUTTI, L., 2005a. Phenology of Tomicus destruens (Wollaston) in northern Italian pine stands, pp. 185-193, in F. Lieutier & D. Ghaioule (eds.). Entomological Research in Mediterranean Forest Ecosystems. INRA Editions, Paris. [ Links ]
FACCOLI, M., PISCEDDA, A., SALVATO, P., SIMONATO, M., MASUTTI, L., BATTISTI, A., 2005b. Phylogeography of the pine shoot beetles Tomicus destruens and T. piniperda (Coleoptera Scolytidae) in Italy. Ann. Forest Sci. 62: 361–368. [ Links ]
FACCOLI, M., BLAŽENEC, M., SCHLYTER, F., 2005c. Feeding response to host and non-host compounds by males and females of the spruce bark beetle Ips typographus in a tunnelling microassay. J. Chem. Ecol. 31: 745–759. [ Links ]
GUERRERO, A., FEIXAS, J., PAJARES, J., WADHAMS, L.J., PICKETT, J.A., WOODCOCK, C.M. 1997. Semiochemically induced inhibition of behavior of Tomicus destruens (Woll.) (Coleoptera Scolytidae). Naturwissenschaften 84: 155–157. [ Links ]
IDZOJTIC, M., KAJBA, D., FRANJIC, J., 2005. Differentiation of F1 hybrids P. nigra J.F. Arnold x P. sylvestris L., P. nigra J.F. Arnold x P. densiflora Siebold et Zucc., P. nigra J.F. Arnold x P. thunbergiana Franco and their parental species by needle volatile composition. Biochem. Syst. Ecol. 33: 427–439. [ Links ]
JACOBSON, M., 1966. Chemical insect attractants and repellents. Ann. Rev. Entomol. 11: 403-422. [ Links ]
JONES, R.L., LEWIS, W.J., BOWMAN,M.C., BEROZA, M., BIERL, B.A., 1971. Host-seeking stimulant for a parasite of the corn earworm: isolation, identification and synthesis. Science 173: 842–843. [ Links ]
KIMMERER, T.W., KOZLOWSKI, T.T., 1982. Ethylene, ethane, acetaldehyde and ethanol production by plants under stress. Plant Phys. 69: 840–847. [ Links ]
KIRKENDALL, L.R., FACCOLI, M., YE, H., 2008. Description of the Yunnan shoot borer Tomicus yunnanensis (Curculionidae, Scolytidae), an unusually aggressive pine shoot beetle from southern China, with a key to all Tomicus. Zootaxa 1819: 25–39. [ Links ]
LÅNGSTRÖM, B., 1980. Distribution of pine shoot beetle attacks within the crown of Scots pine. Studia For. Suec. 154: 1–25. [ Links ]
LÅNGSTRÖM, B., 1983. Life cycles and shoot-feeding of the pine shoot beetles. Studia For. Suec. 163: 1–29. [ Links ]
LANNE, B.S., SCHLYTER, F., BYERS, J.A., LÖFQVIST, J., LEUFVÉN, A., BERGSTRÖM, G., VAN DER PERS, J.N.C., UNELIUS, R., BAECKSTROM, P., NORIN, T., 1987. Differences in attraction to semiochemicals present in sympatric pine shoot beetles Tomicus minor and T. piniperda. J. Chem. Ecol. 13: 1045–1067. [ Links ]
MACCHIONI, F., CIONI, P.L., FLAMINI, G., MORELLI, I., MACCIONI, S., ANSALDI, M., 2003. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour Fragr. J. 18: 139–143. [ Links ]
PERTTUNEN, V., OKSANEN, H., KANGAS, E., 1970. Aspects of the external and internal factors affecting the olfactory orientation of Blastophagus piniperda (Coleoptera, Scolytidae). Contrib. Boyce Thompson Inst. 24: 293–297. [ Links ]
POLAND, T.M., DE GROOT, P., BURKE, S., WAKARCHUK, D., HAACK, R.A., NOTT, R., 2004. Semiochemical disruption of the pine shoot beetle, Tomicus piniperda (Coleoptera: Scolytidae). Environ. Entomol. 33: 221–226. [ Links ]
SABBATINI PEVERIERI, G., FAGGI, M., MARZIALI, L., PANZAVOLTA, T., BONUOMO, L., TIBERI, R., 2004. Use of attractant and repellant substances to control Tomicus destruens (Coleoptera: Scolytidae) in Pinus pinea and P. pinaster pine forests of Tuscany. Entomologica 38: 91–102. [ Links ]
SCHLYTER, F., BIRGERSSON, G., LEUFVÉN, A., 1989. Inhibition of attraction to aggregation pheromone by verbenone and ipsenol. Density regulation mechanisms in bark beetle Ips typographus. J. Chem. Ecol. 15: 2263–2277. [ Links ]
SCHRÖEDER, L.M., EIDMANN, H.H., 1987. Gallery initiation by Tomicus piniperda (Coleoptera: Scolytidae) on Scots pine trees baited with host volatiles. J. Chem. Ecol. 13: 1591–1599. [ Links ]
SCHRÖEDER, L.M., LINDELÖW, A., 1989. Attraction of scolytids and associated beetles by different absolute amounts and proportions of alpha-pinene and ethanol. J. Chem. Ecol. 15: 807–817. [ Links ]
STURGEON, K.B., 1979. Monoterpene variation in ponderosa pine xylem resin related to western pine beetle predation. Evolution 33: 803–814. [ Links ]
TASIN, M., ANFORA, G., IORIATTI, C., CARLIN, S., DE CRISTOFARO, A., SCHMIDT, S., BENGTSSON, M., VERSINI, G., WITZGALL, P., 2005. Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J. Chem. Ecol. 31: 77–87. [ Links ]
VASCONCELOS, T., BRANCO, M., GONCALVES, M., CABRAL, M.T., 2005. Periods of flying activity of Tomicus spp. in Portugal, pp. 177–184, in F. Lieutier & D. Ghaioule (eds.). Entomological Research in Mediterranean Forest Ecosystems. INRA Editions, Paris. [ Links ]
VET, L.E.M., VAN LENTEREN, J.C., HEYMANS, M., MEELIS, F., 1983. An airflow olfactometer for measuring olfactory responses of hymenopterous parasitoids and other small insects. Phys. Entomol. 8: 97–106. [ Links ]
VRKOCOVA, P., VALTEROVA, I., VRKOC, J., KOUTEK, B., 2000. Volatiles released from oak, a host tree for the bark beetle Scolytus intricatus. Biochem. Syst. Ecol. 28: 933–947. [ Links ]
WALLIN, K.F., RAFFA, K.F., 2000. Influences of host chemicals and internal physiology on the multiple steps of post-landing host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environ. Entomol. 29: 442–453. [ Links ]
WOOD, D.L., 1982. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Ann. Rev. Entomol. 27: 411–446. [ Links ]
ZAR, J.H., 1999. Biostatistical Analysis. Prentice Hall Press, New Jersey. [ Links ]
ZHANG, Q.-H., LIU, G., SCHLYTER, F., BIRGERSSON, G., ANDERSON, P., VALEUR, P., 2001. Olfactory responses of Ips duplicatus from inner Mongolia, China to nonhost leaf and bark volatiles. J. Chem. Ecol. 27: 995–1009. [ Links ]
ZHANG, Q.-H., SCHLYTER, F., 2004. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agr. For. Entomol. 6: 1–20. [ Links ]
ZHANG, Q.-H., SCHLYTER, F., LIU, G., SHENG, M., BIRGERSSON, G., 2007. Electrophysiological and Behavioral Responses of Ips duplicatus to Aggregation Pheromone in Inner Mongolia, China: Amitinol as a Potential Pheromone Component. J. Chem. Ecol. 33: 1303–1315. [ Links ]
ZHAO, T., LI, L.S., ZHOU, N., 2002: The attraction of Yunnan pine to pine shoot beetle and tree volatile composition. J. Northeast For. Univ. 30: 47-79. [ Links ]
Acknowledgments
We are grateful to Silvia Carlin and Francesco Nazzi for providing technical assistance and laboratory facilities. The research was partially funded by the Autonomous Province of Trento (SafeCrop Project).













