Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Silva Lusitana
versão impressa ISSN 0870-6352
Silva Lus. vol.22 no.1 Lisboa jun. 2014
ARTIGOS
Factors Affecting the Dispersion of Biscogniauxia mediterranea in Portuguese Cork Oak Stands
Fatores que afetam a dispersão de Biscogniauxia mediterranea nos montados em Portugal
Facteurs affectant la dispersion de Biscogniauxia mediterranea en peuplements de chêne-liège au Portugal
*Joana Henriques, *Maria João Barrento, *Luís Bonifácio, **Alberto Azevedo Gomes, ***Arlindo Lima and **Edmundo Sousa
*Research grant-holder E-mail: joana.henriques@iniav.pt, mjoao.barrento@iniav.pt, luis.bonifacio@iniav.pt
**Senior Researcher, Instituto Nacional de Investigação Agrária e Veterinária, IP., Unidade Estratégica de Investigação de Sistemas Agrários e Florestais e Sanidade Vegetal. Quinta do Marquês, 2780-159-OEIRAS. E-mail: alberto.gomes@iniav.pt; edmundo.sousa@iniav.pt
***Professor, Instituto Superior de Agronomia. Centro de Engenharia dos Biossistemas, Universidade de Lisboa. Tapada da Ajuda, 1349-017 LISBOA. E-mail: arlindolima@isa.utl.pt
ABSTRACT
Charcoal canker is a frequent Quercus suber disease that nowadays contributes to its decline in Portugal. It is caused by Biscogniauxia mediterranea,a wide dispersed endophytic fungus that may be present in symptomless trees, becoming more aggressive in susceptible stressed hosts. This work aims to clarify the fungus dispersal by airborne dispersion and transmission from a tree to its progeny through infected tissues. Thus a Hirst spore-trap and meteorological station were installed in the forest. The results confirmed that ascospores spread as airborne inoculum in natural conditions varying throughout the year. Precipitation is the main factor for ascospores release and wind the way of dispersal. The transmission of the fungus by infected tissues was evaluated in acorns and seedlings from declined and asymptomatic trees, in natural regeneration and in stump sprouting regeneration from infected trees. Although B. mediterranea is present in the seeds, it was not found in seedlings, indicating there is no vertical transmission of the disease. It was also found in natural regeneration in a reduced rate, not due to seed contamination but by early aerial infection. In stump sprouting the fungus was not detected, so it can be used to replace trees.
Key word: acorn, charcoal canker disease, meteorological conditions, natural regeneration, Quercus suber, stump sprout
RESUMO
O carvão do entrecasco é uma doença frequente de Quercus suber que atualmente contribui para o seu declínio em Portugal. Esta doença é provocada por Biscogniauxia mediterranea, um fungo endófito que pode estar presente em árvores assintomáticas, tornando-se agressivo em hospedeiros sob stress. Este trabalho tem como objetivo esclarecer a dispersão do fungo por via aérea e a transmissão através de tecidos infetados da árvore para a descendência. Assim, foram instalados no montado um aparelho capta-esporos tipo Hirst e uma estação meteorológica. Os resultados confirmaram que ascósporos dispersam-se no ar em condições naturais variando ao longo do ano. A precipitação é o principal fator para a libertação dos ascósporos e o vento o meio de dispersão. A transmissão do fungo por tecidos infetados foi avaliada em bolotas e plântulas de árvores degradadas e assintomáticas, em regeneração natural e em rebentamentos de toiça de árvores infetadas. Apesar de B. mediterranea estar presente nas sementes, não foi detetada nas plântulas, indicando que não há transmissão vertical da doença. Foi encontrado em taxa reduzida na regeneração natural, não pela contaminação de sementes, mas por infeção aérea precoce. Nos rebentamentos de toiça o fungo não foi detetado, pelo que poderão ser utilizados para substituição de árvores degradadas.
Palavras-chave: bolota, carvão do entrecasco, condições meteorológicas, Quercus suber, rebentamentos de toiça, regeneração natural
RÉSUMÉ
Le charbon-de-la-mère est une maladie fréquente du Quercus suber entrainant actuellement son déclin au Portugal. Cette maladie est causée par Biscogniauxia mediterranea, un champignon endophyte qui peut être présent dans les arbres asymptomatiques. Il devient plus agressif chez les hôtes sous stress. Ce travail vise à clarifier la dispersion du champignon dans l'air et la transmission à travers des tissus infectés de l'arbre à la descendance. Ainsi, un piège à spores Hirst et une station météorologique ont été installés dans la subéraie. Les résultats ont confirmé que les ascospores sont dispersées dans l'air dans les conditions naturelles variant tout au long de l'année. La précipitation est le principal facteur pour la libération des ascospores et le vent le milieu de la dispersion. La transmission du champignon par les tissus infectés a été évaluée dans les glands et les semis d'arbres dépérissants et asymptomatiques, dans la régénération naturelle et des rejets de souche des arbres morts contaminés. Bien que B. mediterranea soit présente dans les glands, il n'a pas été trouvé dans les semis, indiquant absence de transmission verticale de la maladie. Il a été trouvé en taux réduit dans la régénération naturelle, pas à cause de la contamination des glands, mais par une infection aérienne prématurée. Dans les rejets de souche le champignon n'a pas été détecté, de sorte qu'ils peuvent être utilisés pour remplacer les arbres dépérissants.
Mots-clés: charbon-de-la-mère, conditions météorologiques, gland, Quercus suber, régénération naturelle, rejets de souche
Introduction
Biscogniauxia mediterranea (De Not.) O. Kuntze is an endophytic fungus, widespread in Portuguese cork oak (Quercus suber L.) forests causing charcoal canker disease, presently considered one of the interveners on cork oak decline. This fungus was described in cork oak in Portugal in 1931, as secondary pathogen, associated with trees in an advanced state of decline. However, recently, numerous younger and not degraded cork oak trees have been suffering a sudden decline exhibiting an atypical expansion of this disease (HENRIQUES et al., 2012).
The fungus can live as an endophyte in all of the aerial organs of the plants and act as a pathogen when the oaks suffer prolonged periods of stress. It induces discoloration of the woody tissues, dieback and stem and branch cankers, progressing to the appearance of characteristic black carbonaceous stromata on dead tissues (SANTOS, 2003). In the stromal surface, mature small papillae shaped protrusions are observed corresponding to perithecial necks. Within perithecia are produced ascospores that are considered the most important propagation units of B. mediterranea. Also conidia and mycelia fragments constitute effective inoculum for fungus dissemination (JIMÉNEZ et al., 2005). A great abundance of propagules is produced on colonized parts of the tree.
The dispersal of inoculum units is essential for plant pathogens to infect new hosts and complete their life cycles, for gene flow and diversification of pathogen populations. It is important to understand dispersal mechanisms in order to devise better methods to detect plant pathogens and control the disease they cause (WEST, 2014). In general, inoculum are transported to susceptible hosts by abiotic and biotic vectors: plant pathogens can be dispersed by air, rain, water or soil, and by vectors such as animals, pollen, various microbes, people and machinery and on infected plant material including seeds (WEST, 2014).
The most relevant factor for B. mediterranea dispersion in the field is related with meteorological conditions. In fact, both discharge and germination of ascospores are influenced by them: the ejection of ascospores is associated with high precipitation or high relative humidity and is greatly reduced during dry period (VANNINI et al., 1996b).
Insects are the main biotic vectors, not only transporting the inoculum but also causing wounds that act as an infection point (JIMÉNEZ et al., 2005). They spread the pathogens in short distances within the fields but also over long distances depending on insects' bioecology (WEST, 2014). Several insects have been identified as B. mediterranea vectors, such as Platypus cylindrus associated to cork oak in Portugal (INÁCIO et al., 2011) and Agrilus spp. and Tropideres spp. associated to Quercus cerris in Italy (VANNINI et al., 1996a). In Spain and Morocco, the presence of Cerambyx spp. was associated with the incidence of the disease in Q. suber, although no evidence proved their action as fungi vectors (MARTÍN et al., 2005).
Other pathway for B. mediterranea spread is manmade operations in managed forests: the fungal spores transported by pruning or cork removal forestry equipment can infect other trees. Also, a bad cork removal practice impedes the natural healing of injuries and facilitates the development of diseases (MONTOYA OLIVER, 1988).
Direct transmission of plant diseases might occur through infected plant tissues including vertical transmission when the pathogen infects the seed progeny of an infected plant (ZABALGOGEAZCOA, 2008) and through root and stump sprouting. B. mediterranea has been found as endophyte in different host tissues and organs including leaves, buds, twigs, branches and trunk, wood and bark (FRANCESCHINI et al., 2002). Nevertheless, there aren't references to studies searching this fungus endophytically on acorns of Quercus spp. questioning the possibility of seed borne transmission, nor in stump sprouts, commonly used in Portuguese managed stands when the stump sprouts in apparently good vegetative and phytosanitary conditions are used to replace felled trees.
The objective of this work is to clarify the dispersal of B. mediterranea in Portuguese cork oak stands conditions, namely by aerial dispersal of ascospores associated with meteorological conditions and the dispersion of the fungus from a tree to its progeny through infected tissues: seeds and stump sprout regeneration.
Methods
Aerial dispersion of ascospores of Biscogniauxia mediterranea associated with meteorological conditions: To monitor B. mediterranea ascospores dispersion in field conditions, a 7 day recording volumetric spore trap (Burkard), described by HIRST (1952), and a meteorological station (HOBOware®Pro) were installed in Herdade das Barradas da Serra, Grândola (Alentejo, Portugal), from 5th July 2011 to 13th December 2012. The data of wind velocity were provided by Escola Profissional de Desenvolvimento Rural de Grândola. The stand is located at 38º11'29.35''N 8º37'12.38''O at 150 m altitude with steep slope relief and southeast exposure. It has a density of 147 trees/ha and an agrosilvopastoral land use management.
Based on logging interval of 30 min, daily and weekly average air temperature, relative humidity and daily and weekly total precipitation were obtained. Daily wind velocity was obtained from 10 min intervals average. Associations between the weekly total numbers of ascospores and the meteorological variables were verified through regression analysis, for the period of October 2011 to October 2012. Association between daily total numbers of ascospores and the daily wind velocity was also verified through regression analysis for the period of September to October 2011. The relation between the number of ascospores and meteorological variables were tested by Pearson's coefficient for linear correlations, or Spearman coefficient for non linear correlations. The effect of temperature on spores release was further studied for the period of October 2011 to October 2012: three classes of weekly average temperature were considered (6–12 ºC, 12–18 ºC and 18–24ºC) that were compared using an one way factor ANOVA and a LSD test for determination of homogeneous groups. In order to clarify the relation of ascospores dispersion with precipitation, two classes were considered: days with precipitation above 0.5 mm (1) and days below (0). Non-parametric Kruskall-Wallis test were used to compare the number of captured ascospores between the number of consecutive days with precipitation above 0.5 mm in three days periods. When significant differences were found, post-hoc paired differences were evaluated with Mann-Whitney U tests. Statistical analyses were performed using STATISTICA 6 software.
Dispersion of Biscogniauxia mediterranea through infected tissues: seeds and stump sprout regeneration: 200 acorns were collected from three asymptomatic trees and 200 from three declined trees in the region of Grândola in November 2012. One hundred acorns of each lot were used for immediate fungi isolation and the other hundred were germinated for further isolation of fungi from the seedlings.
Acorns were cut into pericarp and seed coat together (protection tissues) and inner seed (embryo and endosperm), using a sample of about 5 mm2 of each part for fungus isolation. All samples were superficially sterilized through immersion in 9% H2O2 for 12 min in the case of protection tissues and 6 min for inner seed, subsequently rinsed five times in sterile water and left to dry on sterile filter paper under aseptic conditions (GONTHIER et al., 2006; LINALDEDDU et al., 2011). All samples were placed in Petri dishes containing Potato Dextrose Agar (PDA, Difco, USA) acidified with lactic acid (1 ml lactic acid 85% / l PDA, PDAA) to avoid bacterial growth and incubated at 25±1°C in darkness. The development of B. mediterranea was assessed after 5 and 10 days of growth.
A second batch of 80 acorns from undifferentiated origin were collected and cut into protection tissues (pericarp and seed coat), embryo and endosperm to clarify the presence of the fungi in the different parts of the inner seed. Samples preparation was as described above with the following times of sterilization in 9% H2O2: 10 min for external tissues, 2 min for embryo and 6 min for endosperm (GONTHIER et al., 2006; LINALDEDDU et al., 2011).
The preparation of the acorns for germination was made in accordance to the International Seed Testing Association protocol (ISTA, 1996). The acorns were previously superficially disinfected with sodium hypochlorite 1.5% for 5 min. The trays were kept in a control climatic chamber at 20ºC, with a photoperiod of 16 l/8 d for 80 days. The portion of the stem between the cotyledon leaves and next leaves of each seedling was sectioned and used for fungi isolation. Samples preparation was as described above with superficial sterilization in 9% H2O2 for 12 min (GONTHIER et al., 2006; LINALDEDDU et al., 2011).
100 samples of asymptomatic natural regeneration of cork oak less than three years old were collected in stands in the region of Grândola. The portion of the stem between the first and second leaf node was sectioned and used for fungi isolation. Samples preparation was as described above with superficial sterilization in 9% H2O2 for 15 min (GONTHIER et al., 2006; LINALDEDDU et al., 2011).
To search for B. mediterranea in stump sprouts data from two study plots installed in a cork oak plantation stand, in Herdade de Corta-Rabos in the municipality of Montemor-o-Novo (Alentejo, Portugal) were used. The plantation was made in 1995; the two plots of 150 trees were installed in 2010 and monitored until 2013. All the trees were characterized, and the sanitary conditions were observed every year, in particular for the presence of charcoal canker disease. 100 samples of asymptomatic stump sprouts less than three years old of cork oak trees that had charcoal canker were collected. The portion of the stem between the first and second leaf node was sectioned and used for fungi isolation. Samples preparation was as described above with superficial sterilization in 9% H2O2 for 15 min (GONTHIER et al., 2006; LINALDEDDU et al., 2011).
Results
Aerial dispersion of ascospores of Biscogniauxia mediterranea associated with meteorological conditions: Ascospores of B. mediterranea were captured by the spore-trap device, over the period of study, with varying amounts throughout the year. Figure 1 represents the data of the daily captures of B. mediterranea ascospores from 5th July 2011 to 13th December 2012 and the daily average temperature, average relative humidity, average wind velocity and total precipitation for the same period (data collection was interrupted due to equipment failure for some periods).
A linear regression model adjusted between the number of ascospores weekly captured and the weekly total precipitation revealed an accuracy of 54%. Pearson's correlation coefficient indicated a positive significant relationship between the two variables (r=0.74, p<0.05, n=43). It was not possible to adjust any model to the relation between the weekly ascospores capture and weekly medium temperature and relative humidity and also to daily ascospores capture and daily wind velocity. However, the Spearman's correlation coefficients obtained indicated a negative significant relation between weekly ascospores capture and average temperature (rs=-0.62, p<0.05, n=43), a positive but not significant relation with relative humidity (rs=0.21, p>0.05, n=43) and a positive significant correlation between daily ascospores capture and wind velocity (rs=0.38, p<0.05, n=43).
The distribution of weekly captures of ascospores in accordance with average temperatures (Figure 2) allowed to distinguish three classes of temperature: 6–12 ºC, 12–18 ºC and 18–24ºC. The result of one way factor ANOVA demonstrated that temperature interval has a significant effect on the release of ascospores (F(2,40)=10.1164; p=0.0003) and LSD test showed that the class of temperature 12-18°C induced the highest release of ascospores ( of ascospores = 116.3333) differing significantly from classes 6–12 ºC ( of ascospores = 34.5000) and 18–24 ºC ( of ascospores = 6.7273).

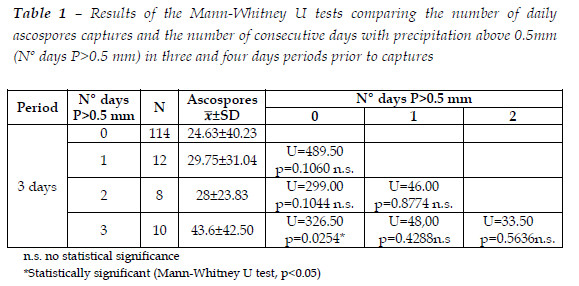
The results of the analysis of relation of the number of daily ascospores captures and number of consecutive days with precipitation above 0.5 mm in three days periods prior to the captures is showed in Table 1. Significant differences were only found between three consecutive days with precipitation below 0.5 mm and three consecutive days with precipitation above it.
Dispersion of Biscogniauxia mediterranea through infected tissues: seeds and stump sprout regeneration: B. mediterranea was isolated both from acorns of declined and asymptomatic trees (Figure 3a). In both cases the fungus was isolated from protection tissues, but it was found in a slightly higher quantity in declined trees: 6% of protection tissues of acorns from declined trees and 4% from asymptomatic trees. In the inner seed tissues the fungus was only found in acorns from declined trees and only in 1% of the samples. However, the fungus was not isolated from any seedling germinated from the acorns batches of asymptomatic and declining trees.
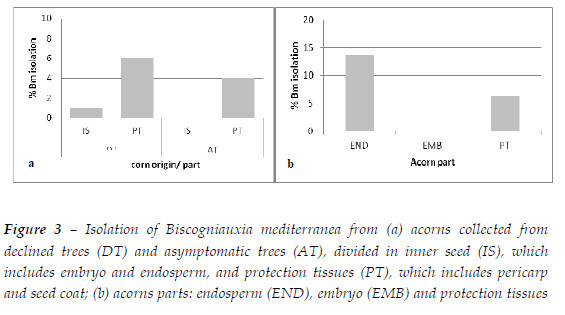
To clarify the presence of B. mediterranea within the acorns and the non-transmission to the seedling, the second batch of acorns from a different origin was evaluated showing that 6% of the samples were infected in the protection tissues and, in the inner seed, 14% were infected in the endosperm but none in the embryo (Figure 3b). In this second batch the rate of infection of the protection tissues was equivalent to acorns from declined trees of the first batch, however, the level of contamination of inner tissues was higher. Nevertheless, the fungus was not detected in the embryo.
From cork oak natural regeneration with less than three years old, B. mediterranea was only isolated in 1% of the samples.
B. mediterranea was not isolated from the samples of asymptomatic sprouts collected from stumps of cork oak trees that had charcoal canker disease.
Discussion
Spore-trapping studies showed that Biscogniauxia mediterranea ascospores spread as airborne inoculum in natural cork oak stand conditions. The dispersal of ascospores occurs throughout the year with a large variation in the amount of spores present in the air, ceasing almost completely during the warm and dry season. Precipitation is the main meteorological variable that influences ascospores dispersion being positively related with it. However, the results demonstrate that significant differences in ascospores discharge are found after a period of three consecutive days with precipitation above 0.5 mm. The consecutive days with precipitation ensure the humidity needed for the ejection of ascospores and to prevent their desiccation until the accommodation in the host and germination.
Our results are in agreement with other authors in the theory that precipitation is the determining factor for the dispersal of ascospores of B. mediterranea. According to VANNINI et al. (1996b), ejection of ascospores is associated with high precipitation or high relative humidity and is greatly reduced during dry periods. JIMÉNEZ et al. (2005) found a positive significant correlation of the amount of sporulation of B. mediterranea with monthly precipitation (mm/day), days with precipitation (regardless of the quantity) and relative humidity. However, the daily and hour level approaches demonstrate that only days with precipitation above 0.5 mm significantly influence the discharge of spores with no direct relationship with the quantity of precipitation. This relationship probably reflects the fact that in the months with more capture of ascospores there were longer periods of time with precipitation, more than the total amount of rainfall.
The temperature is negatively related with ascospores dispersion as was also demonstrated by JIMÉNEZ et al. (2005) although VANNINI et al. (1996b) argued that average monthly temperatures do not affect B. mediterranea ascospores discharge. In fact, during the warmer season of 2012 the ascospores release ceased. The summer of 2011 was particularly rainy in the Alentejo region (IPMA, 2011a, 2011b) so that despite high temperatures there was dispersal of ascospores. These observations allow us to conclude that although both the temperature and precipitation significantly influence the dispersion of spores, precipitation is the most determining factor for the effect.
Although the results have not shown a significant correlation of ascospores discharge and relative humidity, it revealed a positive tendency which was expected as the humidity increases with precipitation. JIMÉNEZ et al. (2005) confirmed the relation of B. mediterranea ascospores captures and average relative humidity.
The wind velocity is also associated with ascospores dispersion as assures their aerial transport. JIMÉNEZ et al. (2005) found a divergence between the hours with precipitation and the hours with spores captures in trap, which shows that although precipitation is needed to cause the discharge of ascospores, the primary vehicle for dispersion of ascospores is the wind, not water, because in the hours with more precipitation the dispersal of ascospores decreased, being significantly higher in subsequent hours.
Transmission of B. mediterranea to new plants from infected Q. suber tissues, seeds and sprouts was not observed in this work. The isolation of B. mediterranea in cork oak acorns was low in asymptomatic and declined trees. In both cases the fungus was found in protection tissues even when it wasn't present in inner tissues which may suggest a superficial infection of the seed due to aerial dispersion of fungus inoculum. Only in acorns from declined trees the fungus was found in inner seed, suggesting the systemic contamination of the seed, nevertheless in neither situation the fungus was found in seedlings of Q. suber germinated from seeds of the same origin. Although the contamination rate of the seeds is low, apparently the fungus does not pass from seed to the plant, which is supported by the results showing that within the seed the fungus was only present in the cotyledons but not in the embryo.
Seed-borne transmission of endophytes is intensively studied on clavicipitaceous endophytes and their grass hosts (SCHARLD et al., 2004), but in tree hosts it is poorly known. Nevertheless, the results presented in this work are in line with others. According to ZABALGOGEAZCOA (2008), horizontal transmission seems to be the predominant mechanism of dispersion among endophytic species. Using RT-PCR techniques, B. mediterranea DNA was not detected on oak epicotyls (Quercus cerris and Quercus ilex) (LUCHI et al., 2005), neither from two weeks old seedlings of Q. cerris generated from surface sterilized acorns in growth chamber, using species specific primers PCR (MAZZAGLIA et al., 2001). Infection studies of Discula quercina, an endophyte of Quercus garryana, showed that despite the presence of the endophyte in the acorn seed coat and cotyledons, it does not appear to grow systemically throughout the plant (WILSON and CARROLL, 1994). Studies in tropical trees showed that seeds and seedlings are virtually endophyte-free, and the incidence of fungal endophytes increases as leaves or seeds grow older, indicating that this dynamics must be driven by horizontal transmission (ARNOLD et al., 2003; GALLERY et al., 2007).
The fact that plants of natural regeneration under three years old might be contaminated by B. mediterranea, even in a very low rate, should also be a result of air-borne dissemination of the fungus in the field. Plant age has an effect upon endophyte diversity and density. As time of exposure to endophyte inoculum increases, plants seem to accumulate an increasing number of endophytes in their tissues. Because of this, older plant parts may harbor more endophytes than younger ones (ZABALGOGEAZCOA, 2008). Nevertheless, it is important to consider that plants might contain B. mediterranea growing endophytically.
All the analyzed stump sprouts were free of B. mediterranea which suggests that the fungus doesn't grow systemically from the base of the trunk or roots to the sprouts. The fungus colonizes bark and woody tissues and of all tree aerial organs but there aren't any references to its presence in the roots in Q. suber or other hosts (FRANCESCHINI et al., 2002). Therefore, with respect to the charcoal canker disease, the use of stump sprouts seems to be a safe practice for the development of the tree which will then be exposed to possible contamination like other trees.
Overall, the primary means of dispersal of B. mediterranea in cork oak stands is by air. The determining factor for the significant release of ascospores is the precipitation, being particularly aggravated in short periods with consecutive days of precipitation above 0.5 mm and mild temperature. The wind velocity is also decisive since it is the vehicle for the dispersal of ascospores. The airborne dispersal of ascospores constitutes an indiscriminate source of inoculum for the trees that can infect all aerial organs including seeds. However, seeds do not constitute a source of contamination since the fungus does not develop systemically to the seedlings. Also the stump sprouts being originally exempted from the fungus can be exploited for the regeneration of trees. In any case, the early contamination of plants/ trees occurs thus being essential the permanent management of the stand aiming a good health and physiological status of the tree.
References
ARNOLD, A.E., MEJÍA, L.C., KYLLO, D., ROJAS, E.I., MAYNARD, Z., ROBBINS, N., HERRE, E.A., 2003. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA5: 15649-15654. [ Links ]
FRANCESCHINI, A, MADDAU, L, MARRAS, F., 2002. Incidence d'endophytes fongiques impliques dans le dépérissement du chêne-liège. IOBC/wprs Bull. 25(5): 29-36. [ Links ]
GALLERY R.A., DALLING J.W., ARNOLD A.E., 2007. Diversity, host affinity and distribution of seed-infecting fungi: a case study with Cecropia. Ecol. 88: 582-588. [ Links ]
GONTHIER, P., GENNARO, M., NICOLOTTI., G., 2006. Effects of water stress on the endophytic mycota of Quercus robur. Fungal Divers. 21: 69-80. [ Links ]
HENRIQUES, J., INÁCIO, M.L., LIMA, A., SOUSA, E., 2012. New outbreaks of charcoal canker on young cork oak trees in Portugal. IOBC/wprs Bull. 76: 85-88. [ Links ]
HIRST, J.M, 1952. An automatic volumetric spore-trap. Ann. Appl. Biol. 36: 257-265. [ Links ]
INÁCIO, M.L., HENRIQUES, J., GUERRA-GUIMARÃES, L., GIL-AZINHEIRA, H., LIMA, A., SOUSA, E., 2011. Platypus cylindrus Fab. (Coleoptera: Platypodidae) transports Biscogniauxia mediterranea, agent of cork oak charcoal canker.Bol. San. Veg. Plagas 37: 181-186. [ Links ]
IPMA – Instituto do Mar e da Atmosfera, I.P., 2011a. Boletim Climatológico Sazonal - Verão 2011. http://www.ipma.pt/resources.www/docs/im.publicacoes/edicoesonline/20110919/FbeyWhFbeyWhOPywMKUD/cli_20110601_20110831_pcl_sz_co_ pt.pdf..
IPMA – Instituto do Mar e da Atmosfera, I.P., 2011b. Boletim Climatológico Sazonal - Outono 2011. http://www.ipma.pt/resources.www/docs/im.publicacoes/edicoesonline/20111216/epTQinKqDBozHUxSqrIQ/cli_20110901_20111130_pcl_sz_co_pt. pdf.
ISTA - International Seed Testing Assotiation, 1996. Seed Sci. & Technol., 24, supplement. Zürich, Switzerland, 335 pp.
JIMÉNEZ, J.J., SÁNCHEZ, M.E., TRAPERO, A., 2005. El chancro carbonoso de Quercus III: Dispersión de ascosporas del agente causal. Bol. San. Veg. Plagas 31: 577-585. [ Links ]
LINALDEDDU, B.T., SIRCA, C., SPANO, D, FRANCESCHINI, A., 2011. Variation of endophytic cork oak associated fungal communities in relation to plant health and water stress. Forest Pathol. 41: 193-201. [ Links ]
LUCHI, N., CAPRETTI, P., PINZAN, I.P., ORLANDO, C., PAZZAGLI, M., 2005. Real-time PCR detection of Biscogniauxia mediterranea in symptomless oak tissue. Lett. Appl. Microbiol. 41: 61-68. [ Links ]
MARTÍN, J., CABEZAS, J., BUYOLO, T., PATÓN, D., 2005. The relationship between Cebambyx spp. Damage and subsequent Biscogniauxia mediterranea infection on Quercus suber forests. Forest Ecol. Manag. 216: 166-174. [ Links ]
MAZZAGLIA, A., ANSELMI, N., GASBARRI, A., VANNINI, A., 2001. Development of Polymerase Chain Reaction (PCR) assay for the specific detection of Biscogniauxia mediterranea living as an endophyte in oak tissues. Mycol. Res. 105: 952-956. [ Links ]
MONTOYA OLIVER, J.M., 1988. Los alcornocales [The oak trees forest]. Madrid. MAPA, 267pp. [ Links ]
SANTOS, M.N.S., 2003. Contribuição para o conhecimento das relações Quercus suber – Biscogniauxia mediterranea (syn. Hypoxilon mediterranea). Silva Lusitana 11(1): 21-29. [ Links ]
SCHARLD, C.L., LEUCHTMANN, A., SPIERING, M.J., 2004. Symbiosis of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 55: 315-340. [ Links ]
VANNINI, A., BIOCCA, M., PAPARATTI, B., 1996a. Contributo alla conoscenza del ciclo biologico di Hypoxylon mediterraneum su Quercus cerris. Inf. Fitopatol. 9: 53-55. [ Links ]
VANNINI, A., PAGANINI, R., ANSELMI, N., 1996b. Factors affecting discharge and germination of ascospores of Hypoxylon mediterraneum (De Not.) Mill. Eur. J. For. Path. 26: 12-24. [ Links ]
WEST, J.S., 2014. Plant Pathogen Dispersal. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net [doi: 10.1002/9780470015902.a0021272] [ Links ]
WILSON, D., CARROLL, G.C., 1994. Infection studies of Discula quercina, an endophyte of Quercus garryana. Mycologia 86(5): 635-647. [ Links ]
ZABALGOGEAZCOA, I., 2008. Review. Fungal endophytes and their interaction with plant pathogens. Span. J. Agric. Res. 6: 138-146. [ Links ]
Submitted for publication in April 2014
Accepted in May 2014
Acknowledgements
Authors are grateful to Doctor Teresa Soares David for coordinating the implementation of the meteorological station, Doctor Lourdes Santos for the assistance in the seed germination and seedling maintenance, Adérito Matos for the help in samples collection, Marina Cardoso and Ricardo Peterson for the help in samples processing, Luís Dias, António Saraiva and Francisco Martins for the help in spore trap and meteorological station installation and management, and Doctor Maria de Lurdes Inácio for manuscript revision. The study plots in Herdade de Corta-Rabos were established in the scope of the protocol INIAV/ Micoflora.This research was supported in part by the Project "Experimentação e Divulgação de Técnicas de Gestão para a Recuperação do Montado de Sobro na Região de Grândola" financed by FFP/ CAP and by a grant from FCT, BD/46787/2008.













