Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.36 no.4 Lisboa out. 2013
ARTIGO
Toxic metals in Raphanus sativus: assessing the levels of cadmium and lead in plants and damage to production
Metais tóxicos em Raphanus sativus: avaliação dos níveis de cádmio e chumbo nas plantas e danos à produção
Marcele Cannata1, Alexandre Bertoli1, Ruy Carvalho1, Ana Rosa Bastos2, Matheus Freitas1, Amanda Augusto1 and Amarílis de Varennes3
1Department of Chemistry, University of Lavras, PO Box 3037, CEP 37200 000, Lavras – MG, Brazil. E-mail: bertolialexandre@yahoo.com.br, author for correspondence
2Department of Soil Science, Federal University of Lavras, PO Box 3037, CEP 37200 000, Lavras – MG, Brazil
3Department Agricultural and Environmental Chemistry, Institute of Agronomy, University of Lisbon, PO Box 1399-017 Lisbon, Portugal
ABSTRACT
An essay in the greenhouse was conducted to evaluate the effects of cadmium and lead in radishes grown in nutrient solution. The doses of Cd used were 0.0; 0.025; 0.1; 0.5 and 1.0 mg L-1 and Pb 0.0; 0.25; 1.0; 5.0 and 10.0 mg L-1. The increase in absorption of Cd and Pb was achieved by increasing the concentration of these elements in the solution. The roots accumulate greater amounts of metal from the leaves, followed by the tubers. The content of Cd deleterious to the production of roots was from 0.1 mg L-1. Higher doses than those used in this study (1.0 mg L-1 Cd and 10.0 mg L-1 Pb) can be considered critical for toxicity and plants would be completely eliminated from such concentrations. Cd and Pb are metals of low translocation in the plants and its harmful effect is due to the deposition of highly stable chelates in the roots.
Keywords: Radish, Nutritive solution, Cadmium, Lead
RESUMO
Um ensaio em casa de vegetação foi realizado para avaliar os efeitos de cádmio e chumbo em rabanetes cultivados em solução nutritiva. As doses de Cd utilizadas foram 0,0; 0,025; 0,1; 0,5 e 1,0 mg L-1 e de Pb 0,0; 0,25; 1,0; 5,0 e 10,0 mg L-1. Os teores de Cd utilizados foram 0; 0.025; 0,1; 0.5 e 1 mg L-1 e Pb 0; 0.25; 1; 5 e 10 mg L-1. O aumento na absorção de Cd e Pb foi obtido com o aumento da concentração destes elementos na solução. As raízes acumularam maiores quantidades de metal do que as folhas, seguido dos tubérculos. O teor de Cd deletério para a produção das raízes foi a partir da dose de 0,1 mg L-1. Doses maiores do que as utilizadas neste estudo (1,0 mg L-1 Cd e 10,0 mg L-1 Pb) podem ser consideradas críticas para a toxicidade, e as plantas seriam completamente eliminadas a partir destas concentrações. Cd e Pb são metais de baixa translocação nas plantas e seu efeito deletério é devido à deposição de quelatos muito estáveis nas raízes.
Palavras-chave: Rabanete, Solução nutritiva, Cádmio, Chumbo
Introduction
The interest for studies about accumulation and toxicity of metals has increased in the last years as a consequence of occupational and environmental exposures, or due to the disturbs caused by these elements, which are induced by special conditions, such as kidney insufficiency (DHaese et al., 1995). In superior organisms, introduction of microelements happens mainly through the respiration system or by means the food chain (Albers et al., 2000; Alberti-Fidanza et al., 2003). Particularly, many elements or dangerous compounds, such as dioxins, pesticides, metals and metalloids accumulate along with the food chain (Rajaratnam et al., 2002). In this way, the food chain is the main entrance for persistent toxic substances. Moreover, in general, these compounds have an anthropogenic origin and, therefore, their amounts in the environment increase with the increase in urban populations, agricultural and industrial emissions (Burger et al., 2002). Hence, the control in the levels of these elements in food is not only an important role for the food quality, but also a system of indirect control of anthropogenic activities and their impact in soil, water and air (Bratakos et al., 2002; Prankel et al., 2004).
The increasing in the worlds population and the tentative of diminishing the agony of billions of people around the whole world lead to different practices and techniques to produce food in order to assure the nutritional necessities of these populations, but not always follows a rigid standard of control about the problem of contamination.
In this work, cadmium (Cd) and lead (Pb) were chosen because they do not have any physiological function, much low tolerance to daily ingestion and are probably the main source of inorganic contaminants to environment and deleterious to living organisms.
The technique in nutritive solution is widely known around the world and its use is increasing in many countries. Its importance is not emerging only due to its applications in horticulture investigation and vegetable production; nutritive solution has also being employed as a tool to solve a large spectrum of problems, including treatments to minimize contamination of soil and underground water, and manipulation of the levels of nutrients in a given product (Castellane and Araujo, 1995).
Because nutritive solution is a homogeneous systems (opposite to soil, which is composed by three phases: soil, liquid and gas), it allows e.g. scrutinized studies about translocation, absorption kinetics and redistribution of minerals in plants (Bell et al., 1991; Qu et al., 2003). In addition, nutritive solution is much more contaminable by heavy metals than soils.
The goals of this work were: a) study the effect of different content of Cd and Pb on the production of radish; b) evaluate the translocation of Cd and Pb in these plants; c) indicate the tolerance limits to Cd and Pb, capable of influencing the production. This work extends to an earlier study on the bean plant (Phaseolus vulgaris), but now related to the analysis of radish (Raphanus sativus) as a common tubercle with important nutritional properties.
Material and Methods
Experiments were carried out in greenhouse at the Federal University of Lavras (Lavras, Brazil) maintained in a greenhouse with an average temperature of 28°C, 11.5 h/ 13 h (winter/summer) photoperiod, and 250-350 µmol m-2 s-1 PAR irradiance (natural radiation reduced with a reflecting mesh) for 45 days, a period corresponding to the vegetative cycle of Radish.
Table 1 shows the components and corresponding amounts of the Clark´s nutritive solution (Clark, 1975), where the mineral concentrations correspond to those standard values for closely related hydroponic systems. Cd (0.0, 0.025, 0.1, 0.5 and 1.0 mg L-1) and Pb (0.0, 0.25, 1.0, 5.0 and 10.0 mg L-1) were added to the nutritive solution, using Cd(NO3)2.4H2O and Pb(NO3)2 as contaminants. These contents were chosen by taking into account those values that intoxicate without eliminate plants during cultivation, as reported elsewhere (Malavolta, 1994), in order to obtain vegetal material enough for laboratorial analysis. Radish (Raphanus sativus L.) was developed in opaque 2 L plastic containers, and the solutions were constantly aerated using plastic tubes and compressor. Seeds were irrigated with demineralized water in order to keep them wet during 18 days. Plants were removed to a Clark´s solution at 25% of the maximum concentration after growing ca. 8 cm, where they were maintained by 5 days. Then, the solution was replaced by another one with 50% of the maximum concentration and, after 5 days, by another solution with 75% of the maximum concentration during 4 days. Replacement of the nutritive solution at 75% of the maximum concentration and the addition of metals were performed once a week, during 3 weeks. The gradual increasing of nutritive solution aimed at adapting the plants to different chemical media; 75% of the maximum concentration was enough for a good cultivation. After finishing the vegetative cycle, plants were separated into shoots and root system, washed with deionized water and dried at 65-70oC until constant mass. The material was grinded and digested using a 2:1 (v/v) nitro-perchloric solution (HNO3:HClO4) (Malavolta et al., 1997).
The content of Cd and Pb of the dry matter of shoots and root system were quantified using a Varian flame absorption spectrophotometer, with acetylene flame and hollow cathode lamp: Cd (228.8 nm, 0.5 crack) and Pb (217 nm, 1.0 crack).
The potential of plants in up taking Cd and Pb from the nutritive solution was measured by the phytoextraction coefficient or transfer index, t, using Equation 1 (Lubben and Sauerbeck, 1991):

The calculations were performed by considering the content of Cd and Pb in shoots plus roots, in all concentrations used; the larger this factor, the larger the contaminant absorption (Henry, 2000).
The relative production index (RP), related to the influence of the metal on the variation of dry matter production, is obtained by Equation 2 (Paiva et al., 2002):

The translocation index (TI) gives the capability of species in translocating Cd and Pb from root to shoots where it is calculated by Equation 3 (Paiva et al., 2002):

The experiment was arranged in a completely randomized factorial (2×5)×4 design (two metals, Cd and Pb, and five dosages for each metal), with four replicates. The average results were submitted to Tukey test (5% probability) using the SISVAR program (Ferreira, 2000).
Results and Discussion
The levels of Cd and Pb applied to nutritive solution influenced the relative production of dry matter, the translocation index and the transfer factor in radish (R. sativus) very differently, discussed as follows.
Cadmium
Relative Production of Dry Matter
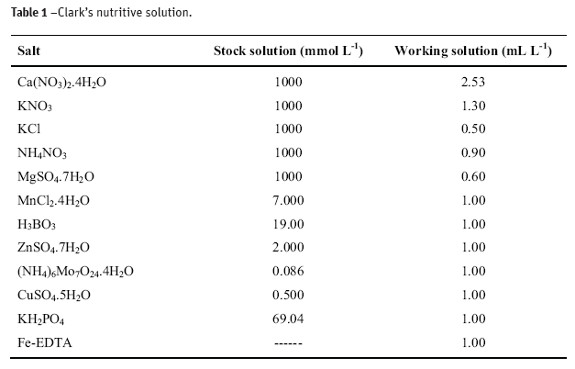
Plots in Figure 1 show the mean data about the growing of radish plants under different levels of Cd. The production of dry matter of radish was clearly compromised (Figure 1) with increasing content of Cd in nutritive solution, and reduction in production of fruits. There was a linear decreasing in the dry matter of leaves and the largest production of tubercle was obtained at the dose 0.1 mg L-1 of Cd. Roots did not exhibit a significant effect to Cd content, with the smallest production of dry matter at the dose 0.1 mg L-1 of Cd. The highest content of metal gave a root production close to the control treatment.
Increasing production of tubercle comparative to the control treatment (47% and 40%) is observed at the doses 0.1 and 0.025 mg L-1 of Cd, respectively. On the other hand, a decrease of 37% between the control treatment and the largest level of Cd (1.0 mg L-1) was observed in the leaves, while such a largest decrease (ca. 36%) was observed at the 0.1 mg L-1 of Cd for roots.
The apparent benefic effect at low doses of Cd (0.025 and 0.1 mg L-1) can be attributed to a competition between Zn (ionic radius 0.74Å) and Cd (ionic radius 0.97Å) (Mahan, 2003). Because Zn2+ is smaller than Cd2+, and under low concentrations of this latter, it is reasonable to assume a larger production of tubercles and leaves larger than the control treatment to a low absorption of Zn2+ and of other essential divalent cations of similar sizes. Small cations like Zn2+ are less probable to combine as electron donors in bulk molecules, such as free amino acids and proteins.
Degenerative effects at the cellular nucleus of leave tissues of radish submitted to Cd have been observed by Vitória et al. (2006); an intense degradation and subsequent rupture of the nuclear wrapper in these plants were observed. This rupture can be due to the increasing of reactive species to oxygen. In plants, such species are produced during mitochondrial reactions, in chloroplasts and peroxysoms, including antioxidant enzymes like catalase and dismutase superoxide, which are important for the metabolism of these reactive species. It has been shown that Cd induces the formation of reactive species to oxygen (Fornazier et al., 2002).
Dismutase superoxides (DSO) are enzymes that catalyze the mutation of O2and HO2+ to H2O2, and can be bonded to a metal (Cu/Zn, Mn and Fe). Plants usually have Cu/Zn-DSO at cytosol, Cu/Zn and/or Fe-DSO at chloroplast, and Mn-DSO at mitochondria. DSOs are important antioxidant agents, but, at high concentrations in animal and bacterial cells, they can induce cell dysfunction and death (Baker et al., 1995). H2O2 can signalize the activation of the antioxidant system in plants, if produced in low concentrations (Gratão et al., 2005).
Prasad (1995) has suggested that Cd is easily absorbed by the root system of plants and translocated by xylem to the aerial portion. Chen et al. (2003) verified that Cd has been mostly absorbed by the root of radish and carrot, but not translocated to the aerial portion in large concentrations.
Content, Translocation and Transfer
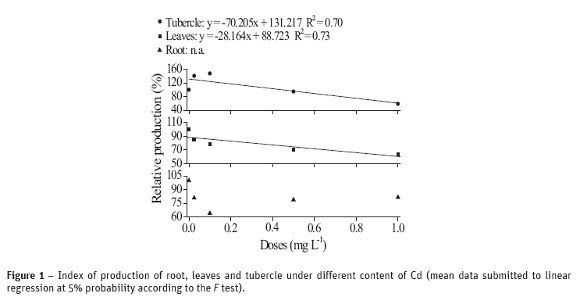
The results of content, translocation and transfer of cadmium absorbed by radish plants are shown in Figure 2. The larger the concentration of metal given to plant, the larger the absorbed and accumulation content of metal in all three compartments (tubercle, leaves and root), Figure 2a. It is worth noting that, when comparing the content of Cd in the different parts, the absorption in roots is 318 times larger from the control treatment to the dose 1.0 mg mL-1 of Cd; the same comparison for leaves and tubercle gives the respective increasing of 100 and 50 times. Accordingly, these data suggest that Cd translocates very poorly throughout the plant, given the important difference of contaminant content among its three compartments. The Cd content in root, stem and leaves were superior to the range 5-30 mg kg-1, which is potentially toxic to plants (Kabata-Pendias and Pendias, 2001).
According to Arduini et al. (1996), the regulation of absorption by the rhizosphere, the accumulation in the roots, in order to preserve the integrity and primary functions, and the low transportation to the aerial portion are considered the possible mechanisms by which the root system can contribute for a tolerance to heavy metals.
The translocation index (TI) given in Figure 2b did not show significant effect as a function of the doses applied in any parts of the plant. A slightly larger value at the 0.025 mg L-1 content was observed, while similar TI values were observed for the remaining levels. The translocations for tubercle and leaves were 12% and 29%, respectively. Overall, Cd translocation did not affect the growing of radish plants.
Adaptation to a variety of stress types is a remarkable characteristic of plants, which have the capacity of activating an antioxidant system when exposed to oxidative stress, keeping the normal development (Droge, 2002); tannins, phenols, alkaloids, lignin, caffeine and amines can act as antioxidants (Okada et al., 1996). In this way, the antioxidant property of eliminating free radicals and/or reactive species and chelating reactive metals is attributed to some phenolic compounds in plants (Droge, 2002). These effects are described when pollutant and toxic substances are used to the activity of antioxidant enzymes in living organisms; heavy metals are listed amongst pollutants (Larson, 1988).
The tubercle in radish contains several antioxidant compounds, including the two main groups of bioactive sulfur compounds: glycosilates and S-methylcysteine sulfoxides (Manach et al., 2004). Thus, it is reasonable to assume that the lower content of Cd in tubercles (Figure 2a) in comparison to leaves is due to a possible elimination of Cd2+ complexed with antioxidant compounds (it is worth remembering that the tubercle develops after the formation of the aerial part).
The transfer of metals from soil or nutritive solution to plants can be evaluated by the transfer factor t (Figure 2b). The larger t, the larger the transfer of metal from soil/nutritive solution to the plant and, consequently, the larger the ability of the vegetable in withdrawing the contaminant from the system (in this case, the nutritive solution). A significant effect of the transfer factor was not found (Figure 2b), i.e. t did not change independent of the level Cd, corroborating the survival mechanism, due to the low capacity of the plant in withdrawing Cd from the system.
Even using balanced content in the nutritive solution, the concentration of Zn (a nutrient that affects directly the plant growing) seems to be low enough to facilitate the absorption of Cd by roots. According to Faquin (2005), there is a competition between Cd and Zn in the absorption by plants, when the Zn concentration is lower than that of Cd.
The effect of Cd in the enzymatic activity is related to the fact that a number of enzymes contain metals and the replacement of these by other metals of same charge and similar size can result in the inhibition of the enzyme activity. Zinc is present in many metalloenzymes, in addition to make part of various transcription factors for the regulation of gene expression. Due to its similarity to Zn, cadmium can replace it, resulting in modification of the enzyme activity (Shaw et al., 2004). According to Kurdziel et al. (2004), Cd modified and dissociated the structure of a given enzyme in some species of plants, probably because of the replacement of structural Mg by Cd.
Decreasing in the enzyme activity, or even its inactivation, can be explained by the reaction Cd2+ + 2(R–SH) ® R–S–Cd–S–R + 2H+ (Baird, 2002); EDTA solution (pH ~ 6,5) is suggested to reverse intoxication of animals poisoned by Cd: Cd(SR)2 + [H2EDTA]2- ® [Cd(EDTA)]2- + 2(R–SH).
Divalent cadmium (Cd2+) is electron deficient (4d10) and has a great ability of combining to electron donor species to form stable complexes. This tendency can occur with electrons from free amino acids, enzymes and proteins, as pointed out by Silva et al. (2007a, b) by using a synthetic amino acid (DTPA, diethylenetriaminopentacetic acid). These works seem to prove the intoxication mechanism of plants by Cd2+ and also the efficiency of EDTA to reverse this intoxication. Chlorosis in leaves, wilting and reduction of growing are symptoms of plants intoxicated by Cd (Alloway, 1990).
The distribution of Cd throughout the plant is related to phytochelatins (Guo and Marschner, 1995); the complex Cd-phytochelatin can represent a mobile form for the transportation of Cd from roots to aerial parts. Phytochelatin is a complex structure, with peptides rich in cysteine, i.e. rich in electron donor amino acids, which is the necessary condition for the formation of chelates with transition metals (Lewis acids), like Cd (Malavolta, 2006).
Lead
The content of lead (Pb) in nutritive solution as the medium to cultivate radish was evaluated as a function of the following parameters.
Relative Production of Dry Matter
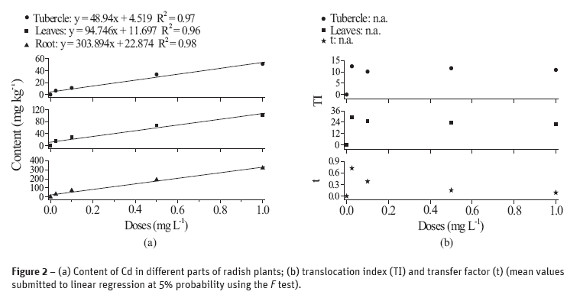
Figure 3 shows the mean data relative to plant growing under different levels of Pb. A linear decreasing in production of tubercles and leaves is observed but there was an increasing in the two lower concentrations of Pb relative to the control treatment, i.e. an increase of 36% and 35% for leaves and tubercle, respectively, in the dose 0.25 mg L-1, and of 17% and 10% at the 1 mg L-1 of Pb. The most important decrease in production was observed at the 10 mg L-1 of Pb: 39% for leaves and 63% for tubercle.
The absorbed Pb accumulates in the cell wall, predominantly in the roots, contributing for diminishing its toxic effect to plant, as well as its transportation to fruits (Faquin, 2005). This seems to explain the benefic effect of some low content of Pb to plants. On the other hand, chlorosis in young leaves of plants treated with Pb, such as observed in arugula, suggests that this element affects the synthesis of chlorophyll and, due to the reduced transport of Fe and formation of heme groups, the deficiency symptoms appear in leaves, causing damages to stressed plants (Fodor et al., 1998; Kupper et al., 1996).
In addition to the inhibition of photosynthesis, Pb alters the mineral nutrition and hydric balance, it modifies the hormonal status and affects the structure and permeability of the membrane (Sharma and Dubey, 2005). The most visible symptoms of toxicity are reduced growth, chlorosis and darkening of the root system, as well as its decreasing (Eun et al., 2000). Concentrations below the toxic level can stimulate root growing (Baligar et al., 1998; Bergmann, 1992).
Content, Translocation and Transfer
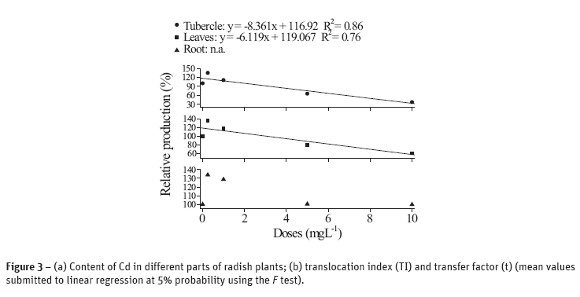
The results of content, translocation and transfer of different content of Pb absorbed by plants are shown in Figure 4. Similarly to that observed for plants which have their growth influenced by cadmium, those samples with Pb have also absorbed more when exposed to larger levels of Pb. In addition, the different parts of the radish plant exhibited different content of Pb (Figure 4a). The content of Pb at the higher concentration was 13 times larger in roots than in tubercle and 20 times larger than in leaves, which reflects the high concentration in roots and, consequently, its low mobility throughout the plant.
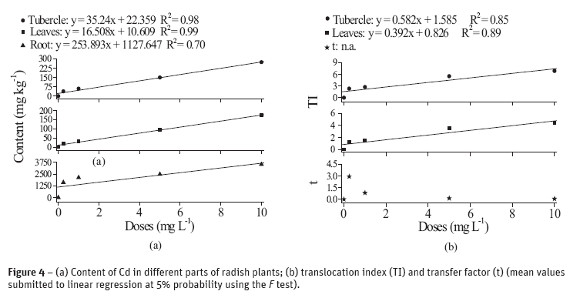
These results converge to the findings of Silva et al. (2007a, b) about the synthesis and characterization of M-DTPA (M = Pb, Cd, Zn and Cu) chelates; according to these authors, these chelates, particularly Pb-DTPA, are structures with high steric hindrance. This supports the hypothesis on the difficulty of Pb in ascending throughout the plant, since it is mostly complexed to free amino acids, proteins or root secretions, which are good electron donors, like DTPA.
The chemical similarity of Cd and Pb in plants, such as between Cu and Zn, has also been detected by Paim et al. (2006) in soil contaminated with waste Zn from mining activities. After Zn up taking from zinc carbonate, the waste rich in Pb, Cd and Zn causes serious environmental damages to soil.
According to Figure 4b TI was affected by the concentration of Pb in solution and that radish, at low concentrations of Pb, increases the metal transfer, i.e. radish has small capability of withdrawing Pb (as well as Cd) from the system and accumulate it in its dry matter.
The absorption of Pb to roots occurs through the plasmatic membrane (probably through calcium channels). Roots are capable of accumulating significant amounts of this metal and, simultaneously, of restricting its translocation to the aerial part (Sharma and Dubey, 2005). Pb moves in the root at low concentrations by apoplast through the cortex and accumulates near endodermis. Endodermis acts as partial barrier for the translocation of Pb from roots to the aerial part. This can be a reason for the substantial acummulation of Pb in roots relative to the aerial part (Verma and Dubey, 2003). According to Kabata-Pendias and Pendias (2001), the mechanism of Pb exclusion is actually the root deposition. This phenomenon is due to the binding of metal to insoluble organic polymers.
Translocation of Pb from roots to aerial parts is limited, in which only 3% of Pb in the roots is translocated to the aerial portion. Only 0.003 to 0.005% of the Pb available in soil is absorbed by plants. Higher accumulation of Pb occurs in foliar plants, like lettuce, which can accumulate up to 0.15% of Pb in the dry matter (Kabata-Pendias and Pendias, 2001).
The better mobility of Cd2+ compared to Pb2+ can be explained by the facility of combining to -SH groups of phytochelatins. The affinity of Cd2+ to S is given by the fact that Cd2+ is a soft Lewis acid and S is a soft Lewis base. Soft acids and bases combine themselves, giving rise to stable structures (Shiriver and Atkins, 2008). Another acceptable argument is the periodic properties of both metals, especially the ionic radius (0.97 Å and 1.32 Å for Cd2+ and Pb2+, respectively), and density (8.6 g cm-3 for Cd and 11.3 g cm-3 for Pb). These factors impose difficulties to mobility and, consequently, to the activity of Pb when compared to Cd. Antoniadis et al. (2007) and Oliveira et al. (2009) attribute the low mobility of Pb to the electronegativity and, thus, Cd (1.7 eV) would be more mobile than Pb (2.3 eV).
Despite radish plants have developed more vigorously at few amounts of Cd and Pb, a phytotoxic content of this metal was found. Thus, it is reasonable to say that content of such metal apparently inoffensive to some plants cannot be to their consumers.
Conclusions
Cadmium (Cd) and lead (Pb) translocate poorly in plants, and their deleterious effects are attributed to root deposition of stable chelates; root deposition of Pb is more likely formed than Cd. In radish, both Cd and Pb accumulate largely in roots, followed by leaves. The plants developed better at 0.250 mg L-1 of Pb in the three parts analyzed. Content of Pb and Cd larger than 10.000 mg L-1 and 1.000 mg L-1, respectively, are critical for toxicity and, from that, plants start to be eliminated.
Acknowledgement
CAPES and CNPq are gratefully acknowledged for the studentship and fellowship (to M.G.C. and M.P.F.).
Bibliographic References
Albers, B.P.; Steindl, H.; Schimmack, W. and Bunzl, K. (2000) - Soil-to plant and plant-to-cows milk transfer of radiocaesium in alpine pastures: significance of seasonal variability. Chemosphere, vol.41, n.5, p. 717-723. [ Links ]
Alberti-Fidanza, A.; Burini, G.; Perriello, G. and Fidanza, F. (2003) - Trace elements intake and status of Italian subjects living in the Gubbio area. Environmental Research, vol.91, n.2, p. 71-77. [ Links ]
Alloway, B.J. (1990) - Cadmium, In: Heavy metals in soils. New York, EUA: J. Willey. [ Links ]
Antoniadis, V.; McKinley, J.D. and Zuhairi, Y.W. (2007) - Single-element and competitive mobility measured with column infiltration and batch tests. Journal of Environmental Quality, vol.36, n.1, p. 53-60. [ Links ]
Arduini, I.; Godbold, D.L. and Onnis, A. (1996) - Cadmium and copper uptake and distribution in Mediterranean tree seedlings. Physiologia Plantarum, vol.97, n.1, p. 111-117. [ Links ]
Baird, C. (2002) - Environmental Chemistry. Porto Alegre, Brazil: Bookman. [ Links ]
Baker, C.J. and Orlandi, E.W. (1995) - Active oxygen in plant pathogenesis. Annual Review of Phytopathology, vol.33, p. 299-321. [ Links ]
Baligar, V.C.; Fageria, N.K. and Elrashidi, M.A. (1998) - Toxicology and nutrient constraints on root growth. Hortscience, vol.33, n.6, p. 960-965. [ Links ]
Bell, P.F.; James, B.R. and Chaney, R.L. (1991) - Heavy-metal extractability in long-term sewagesludge and metal salt-amended soils. Journal of Environmental Quality, vol.20, n. 4, p. 481-486. [ Links ]
Bergmann, W. (1992) - Nutritional disorderes of plants: developments, visual and analytical diagnosis. New York, EUA: G. Fischer. [ Links ]
Bratakos, M.S.; Lazos, E.S. and Bratakos, S.M. (2002) - Chromium content of selected greek foods. Science of the Total Environment, vol.290, n. 1, p. 47-58. [ Links ]
Burger, J.; Gaines, K.F.; Boring, C.S.; Stephens, W.L.; Snodgrass, J.; Dixon, C.; McMahon, M.; Shukla, S.; Shukla, T. and Gochfeld, M. (2002) - Metal Levels in Fish from the Savannah River: Potential Hazards to Fish and other Receptors. Environmental Research, vol.89, n. 1, p. 85-97. [ Links ]
Castellane, P.D. and Araujo, J.A.C. (1995) - Cultivo sem solo, hidroponia. Jaboticabal, Brazil: Funesp. [ Links ]
Chen, Y.X.; He, Y.F.; Yang, Y.; Yu, Y.L.; Zheng, S.J.; Tian, G.M.; Luo, Y.M. and Wong, M.H. (2003) - Effect of cadmium on nodulation and N2-fixation of soybean in contaminated soils. Chemosfere, vol.50, n. 6, p. 781-787. [ Links ]
Clark, R.B. (1975) - Characterization of phosphatase of intact maize roots. Journal of Agriculture and Food Chemistry, vol.23, p. 458-460. [ Links ]
DHaese, P.C.; Landeghem, G.F.V.; Lamberts, L.V. and De Broe, M.E. (1995) - HPLC-AAS hybrid technique for studying the speciation of trace metals (Al, Fe, Si, Hg) in biological fluids. Microchimica Acta, vol.120, n. 1-4, p. 83-90. [ Links ]
Droge, W. (2002) - Free radicals in the physiological control of cell function. Physiological Review, vol.82, n. 1, p. 47-95. [ Links ]
Eun, S.O.; Youn, H.S. and Lee, Y. (2000) - Lead disturbes microtube organization in the root meristem of Zea mays. Physiology Plantarum, vol.110, n. 3, p. 357-365. [ Links ]
Faquin, V. (2005) - Nutrição mineral de plantas. Lavras, Brazil: Ufla/Faepe. [ Links ]
Ferreira, D.F. (2000) - Análises estatísticas por meio do Sisvar para Windows versão 4.0. In 45th Annual Meeting of the Brazilian Region in the International Society of Biometrics. UFSCar, São Carlos, p. 255-258. [ Links ]
Fodor, F.; Cseh, E.; Varga, A. and Záray, G. (1998) - Lead uptake, distribution, and remobilization in cucumber. Journal of Plant Nutrition, vol.21, n. 7, p. 1363-1373. [ Links ]
Fornazier, R.F.; Ferreira, R.R.; Pereira, G.J.G.; Molina, S.M.G.; Smith, R.J.; Lea, P.J. and Azevedo, R.A. (2002). Cadmium stress in sugar cane callus cultures: effect on antioxidant enzymes. Plant Cell, Tissue and Organ Culture, vol.71, p. 125-131. [ Links ]
Gratão, P.L.; Prasad, M.N.V.; Cardoso, P.F.; Lea, P.J. and Azevedo, R.A. (2005) - Phytoremediation: green technology for the clean up of toxic metals in the environment. Brazilian Journal of Plant Physiology, vol.17, n. 1, p. 53-64. [ Links ]
Guo, Y. and Marschner, H. (1995) - Uptake, distribuition, and binding of cadmium and nickel in different plant species. Journal of Plant Nutrition, vol.18, p. 2691-2706. [ Links ]
Henry, J.R. (2000) - An overview of the phytoremediation of lead and mercury. Washington, EUA: PA. [ Links ]
Kabata-Pendias, A. and Pendias, H. (2001) - Trace elements in soil and plants. Boca Raton, EUA: CRC Press. [ Links ]
Kupper, H.; Kupper, F. and Spiller, M. (1996) - Environmental relevance of heavy metal-substituted chrophylls using the example watter plants. Journal of Experimental Botany, vol.47, n. 2, p. 259-266. [ Links ]
Kurdziel, B.M.; Prasad, M.N.V. and Strzalka, K. (2004) - Photosynthesis in heavy metal stressed plants. New York, EUA: G. Fischer. [ Links ]
Larson, R.A. (1988) - The antioxidantes of higher plants. Phytochemistry, vol.27, n.4, p. 969-978. [ Links ]
Lubben, S. and Sauerbeck, D. (1991) - The uptake and distribution of heavy-metals by spring wheat. Water Air and Soil Pollution, vol. 57, n. 8, p. 239-247. [ Links ]
Mahan, B.M. (2003) - Química: um curso universitário. São Paulo, Brazil: E. Blucher. [ Links ]
Malavolta, E. (1994) - Fertilizantes e seu impacto ambiental: micronutrientes e metais pesados mitos, mistificação e fatos. São Paulo, Brazil: Produquímica. [ Links ]
Malavolta, E. (2006) - Manual de nutrição mineral de plantas. São Paulo, Brazil: Ceres. [ Links ]
Malavolta, E.; Vitti, C.C. and Oliveira, S.A. (1997) - Avaliação do estado nutricional das plantas. Piracicaba, Brazil: Esalq-USP. [ Links ]
Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C. and Jiménez, L. (2004) - Polyphenols: food sources and biovailability. The American Journal of Clinical Nutrition, vol.79, n. 5, p. 727-747. [ Links ]
Okada, Y.; Kaneko, M. and Okajima, H. (1996) - Hidroxyl radical scavenging activity of naturally occurring furanfatty acids. Biological and Pharmaceutical Bulletin, vol.19, n. 12, p. 1607-1610. [ Links ]
Oliveira, R.C.; Araújo, A.R.; Carvalho, R.; Guilherme, L.R.G.; Passos, L.P. and Marques, J.J. (2009) - Movimento de zinco em colunas de solo tratados com resíduo de calcário oriundo de mineração. Revista Ceres, vol.56, n. 5, p. 679-684. [ Links ]
Paim, L.A.; Carvalho, R.; Abreu, C.M.P. and Guerreiro, M.C. (2006) - Estudo dos efeitos do silício e do fósforo na redução da disponibilidade de metais pesados em área de contaminação. Química Nova, vol. 29, n. 1, p. 28-33. [ Links ]
Paiva, H.N.; Carvalho, J.G. and Siqueira, J.O. (2002) - Índice de translocação de nutrientes em mudas de cedro (Cedrela fissilis Vell.) e de ipê-roxo (Tabebuia impetiginosa Mart. Standl.) submetidas a doses crescentes de cádmio, níquel e chumbo. Revista Árvore, vol. 26, n. 4, p. 467-473. [ Links ]
Prankel, S.H.; Nixon, R.M. and Phililips, C.J.C. (2004) - Meta-analysis of Feeding Trials Investigating Cadmium Accumulation in the Livers and Kidneys of Sheep. Environmental Research, vol.94, n. 2, p. 171-183. [ Links ]
Prasad, M.N V. (1995) - Cadmium toxicity and tolerance in vascular plants. Environmental Experimental Botany, vol.35, n. 4, p. 525-545. [ Links ]
Qu, R.L.; Li, D.; Du, R. and Qu, R. (2003) - Lead uptake by roots of four turfgrass species in hydroponic cultures. Hortscience, vol.38, n. 4, p. 623-626. [ Links ]
Rajaratnam, G.; Winder, C. and An, M. (2002) - Metals in drinking water from new housing estates in the Sydney area. Environmental Research, vol.89, n. 2, p. 165–170. [ Links ]
Sharma, P. and Dubey, R.S. (2005) - Lead toxicity in plants. Brazilian Journal Plant Physiology, vol.17, n. 1, p. 35-52. [ Links ]
Shaw, B.P.; Sahu, S.K. and Mishra, R.K. (2004) - Heavy metal induced oxidative damage in terrestrial plants. New York, EUA: G. Fischer. [ Links ]
Shriver, D.F. and Atkins, P.W. (2008) - Química inorgânica. Porto Alegre, Brazil: Bookman. [ Links ]
Silva, V.L.; Carvalho, R.; Freitas, M.P.; Tormena, C.F. and Melo, W.C. (2007a) - Spectrometric and theoretical investigation of the structures of Cu and Pb/DTPA complexes. Structural Chemistry, vol.18, n. 5, p. 605-609. [ Links ]
Silva, V.L.; Carvalho, R.; Freitas, M.P.; Tormena, C.F. and Melo, W.C. (2007b) - Structural determination of Zn and Cd-DTPA complexes: MS, infrared, 13C NMR and theoretical investigation. Spectrochimica Acta, vol.68, n. 5, p. 1197-1200. [ Links ]
Verma, S. and Dubey, R.S. (2003) - Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science, vol.164, n.4, p. 645-655. [ Links ]
Vitória, A.P.; Cunha, M. and Azevedo, R.A. (2006) - Ultrastructural changes of radish leaf exposed to cadmium. Environmental and Experimental Botany, vol.58, p. 47-52. [ Links ]
Recebido/Received: 2013.05.28
Aceitação/Accepted: 2013.09.27














