Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.36 no.4 Lisboa out. 2013
ARTIGO
A climate change scenario and soil ammonium fixation during the seasonal rice (Oryza sativa) growth in Portugal under intermittent flooding
A mudança climática na fixação do amónio no solo durante o ciclo cultural de arroz (Oryza sativa) sob alagamento intermitente, em Portugal
Nuno Figueiredo1, Corina Carranca2,*, João Coutinho3, Henrique Trindade4, José Pereira4,5, Paula Marques6 e Amarilis de Varennes7,*
1 Unidade Estratégica de Investigação e Serviços Sistemas Agrários e Florestais e Sanidade Vegetal (UEIS-SAFSV), Instituto Nacional de Investigação Agrária e Veterinária (INIAV), Ministério da Agricultura e do Mar, 2784-505 Oeiras, Portugal. E-mail: figueiredo.nun@gmail.com
2 Unidade Estratégica de Investigação e Serviços Sistemas Agrários e Florestais e Sanidade Vegetal (UEIS-SAFSV), Instituto Nacional de Investigação Agrária e Veterinária (INIAV), Ministério da Agricultura e do Mar, Quinta do Marquês, Av. República, Nova Oeiras, 2784-505 Oeiras, Portugal. E-mail: corina.carranca@iniav.pt, author for correspondence
3 Centro de Química, Escola de Ciências da Vida e do Ambiente (ECVA), Universidade de Trás-os-Montes e Alto Douro (UTAD), 5001-801 Vila Real, Portugal. E-mail: j_coutin@utad.pt
4 Centro de Investigação e Tecnologias Agroambientais e Biológicas (CITAB), Escola de Ciências Agrárias e Veterinárias (ECAV), Universidade de Trás-os-Montes e Alto Douro (UTAD), 5001-801 Vila Real, Portugal. E-mail: htrindad@utad.pt
5 Departamento de Zootecnia, Engenharia Rural e Veterinária (DZERV), Escola Superior Agrária de Viseu (ESAV), Instituto Politécnico de Viseu (IPV), 3500-606 Viseu, Portugal. E-mail: jlpereira@esav.ipv.pt
6 Centro Operativo e Tecnológico do Arroz (COTArroz), 2120-014 Salvaterra de Magos, Portugal. E-mail: pmarques@cotarroz.pt
7 Departamento de Química e Ambiente, Instituto Superior de Agronomia (ISA) Universidade de Lisboa (UL), Tapada da Ajuda, Lisboa, Portugal. E-mail: adevarennes@gmail.com
ABSTRACT
The newly fixed NH4+ in clay minerals should be considered for an efficient management of plant nutrition. In a clay-loam soil cultivated in 2012 with rice under intermittent flooding and conventional agronomic practices, the dynamics of pH, N-inorganic and non-exchangeable NH4+ was evaluated i) under field conditions, air temperature and atmospheric [CO2] (375 µmol mol-1 air) in Salvaterra de Magos (Portugal), and ii) under elevated temperature and temperature+[CO2] in controlled microclimate. For that, open-top chambers were used to simulate the conditions of an increased mean air temperature (2-3 °C), alone or combined with increased [CO2] (550 µmol mol-1 air). Non-exchangeable NH4+ was significantly higher under open-field conditions compared with the temperature elevation, with or without elevated atmospheric [CO2]. Temperature elevation reduced the fixation rate of the cation, while the CO2 concentration rise did not affect particularly the non-exchangeable form. Further studies are required for consolidation of these findings together with microbial communities and dynamics of C and N in soil.
Keywords: Atmospheric CO2, clay loam soil, controlled environment, open-field, temperature.
RESUMO
O NH4+ recém-fixado nos minerais argilosos deve ser considerado numa gestão eficiente da nutrição vegetal. Num solo argilo-limoso, localizado em Salvaterra de Magos (Portugal), cultivado em 2012 com arroz, sob alagamento intermitente e práticas agronómicas convencionais, avaliou-se a dinâmica do pH, N-inorgânico e NH4+ não-trocável i) em condições de campo, de temperatura do ar e [CO2] atmosférico (375 µmol mol-1), -, e de ii) aumento da temperatura e temperatura+[CO2] em microclima controlado. Foram utilizadas câmaras de topo aberto para simulação do aumento da temperatura média do ar (2-3 ºC), apenas ou combinado com aumento da [CO2] (550 µmol mol-1). Os resultados mostraram que o NH4+ fixado foi superior em condições de campo, em comparação com a elevação da temperatura, com ou sem elevação da [CO2]. A elevação da temperatura reduziu a taxa de fixação do catião, enquanto o aumento da [CO2] não afectou o NH4+ fixado. Mais estudos são necessários para a consolidação destes resultados, bem como da dinâmica do C, N e população microbiana no solo.
Palavras-chave: Ambiente controlado, ar livre, concentração deCO2, solo argilo-limososo, temperatura.
Introduction
Rice is a staple food for nearly half of the worlds population, most living in developing countries. Portugal is the first rice consumer per capita in Europe and the fourth producer (6 t ha-1 in 28,000 ha), contributing to the 5.3% of the total European production. Rice cultivation in Portugal is mostly located in the central and southern regions (Mondego, Tagus and Sado valleys) as a monoculture under flooding or intermittent flooding regime. The height of floodwater varies considerably during the season depending on plant size, climatic conditions, soil type and agronomic practices.
Simultaneous supply of ammonium (NH4+) and nitrate (NO3-) can improve rice growth (Shi et al., 2010), but NH4+ is the preferred form of nitrogen (N) for an efficient rice absorption and microbial assimilation under flooded conditions. Ammonium transformation in the paddy rice field is an important process with respect to both agricultural production and environmental protection. Flooding enhances ammonification by facultative anaerobic microorganisms, providing NH4+ to thesoil solution. This cation is then adsorbed or fixed (non-exchangeable form) in the clay minerals, especially the 2:1 expansive minerals (e.g. illite, vermiculite, smectite). This non-exchangeable cation in soils rich in clay minerals contributes markedly to rice nutrition. The effects of fertilization on NH4+ sequestration in soils have been reported mostly for upland unflooded soils (e.g. Carranca, 1996). Few studies have been made on paddy soils, and the underlying mechanisms for NH4+ exchange are far less understood with contradictory reports (Scherer and Zhang, 1999; Liu et al., 2008).
Ammonium is captured by the contracted interlayers of clay minerals after addition of N fertilizers (newly fixed NH4+) and becomes temporarily unavailable for crop nutrition. In flooded rice soils, the anoxic conditions following soil submergence and the accumulation of NH4+ are favorable for the temporary fixation of this N form in soils with high amounts of expandable 2:1 clay minerals. The newly fixed NH4+ is thus protected from N losses via nitrification, denitrification and volatilization processes which may occur during the drying and rewetting of paddy rice soils under the intermittent water regime. In waterlogged soils, NH4+ fixation is stimulated by flooding because soil organic carbon enhances the decline in redox potential (Eh) (Schneiders and Scherer, 1998) which induces a reduction of octahedral iron (Fe) in 2:1 clay minerals, and therefore an increase in the negative charge with a simultaneous higher Coulombic attraction, i.e. higher affinity between the interlayer cations and the silicate layers (Scherer and Zhang, 1999, 2002; Liu et al., 2008). Therefore Eh may have an important impact on the fate of NH4+ in paddy soils. Further, coating of the surface of clay minerals by Fe oxides also has an impact on the diffusion of NH4+ into or out of the interlayers of clay minerals (Scherer and Zhang, 1999). Therefore, the reversible oxidation and reduction of Fe oxides in paddy rice soils is a mechanism of special importance in sorption/fixation and desorption/diffusion of NH4+ and its availability to rice.
The rate of fixation is rapid, with the proportion of fixed NH4+-N:total N ≥11% in cultivated soils (Carranca, 1996; Qiu et al., 2012). The recently fixed NH4+ can be released into the soil solution when the clay interlayers expand and the NH4+ in the soil solution falls below a certain level, becoming available to plants and soil microorganisms. The release of NH4+ is highest in the rhizosphere of rice plants, by the presence of oxygen (O2) and/or NO3- and decreases with distance from the roots, correlating well with the Eh (Zhang and Scherer, 2002). The fixation and subsequent release of recently fixed NH4+ from fertilizer N is of importance in its uptake by plants and differs remarkably with soil texture, clay mineral composition, pH, temperature, fertilization practice, and cropping system (Carranca, 1996; Lu et al., 2010). Results on the availability of non-exchangeable NH4+ for crops are contradictory and are still open to discussion.
The climate change has increased the atmospheric carbon dioxide concentration ([CO2]) from 280 to 380 µmol mol-1 of air in the past two centuries, and is expected to reach 700-1000 µmol mol-1 of air until 2100 if no mitigation measures to reduce CO2 emissions are accounted for (IPCC, 2007). In addition, the rise in the global average air temperature will be between 1.8 to 4.0 ºC by the end of the present century relative to the mean value for 1980-1999. Scenarios of climate change by temperature and CO2 elevation can be particularly relevant in Mediterranean regions. The continuing increase in atmospheric [CO2] and projections of possible future increases in global air temperature caused by the [CO2] elevation have stimulated scientific interest in the effects of these climate variables on important food crops, but literature on soil N, C and microbial dynamics is missing. As to the temperature effect per se literature is contradictory sometimes reporting that fixation of NH4+ in soils increases with the temperature rise by the dehydration and contraction of the crystal lattice. Juang (1990) observed that treatments with temperatures at 25 °C, 40 °C and 70 °C had little influence on the non-exchangeable NH4+ in both the soil and clay, while at 110 °C, the fixed cation in the clay increased significantly, by 13% in a Latosol and 99% in a recent sandstone-shale alluvial soil. This indicates that a temperature of 110 °C may remove more water molecules from the interlayer of the clay minerals, thus increasing the amount of fixed NH4+ in the clay fraction. By contrast, other authors related that fixation is stronger below 30 ºC, and even more by freezing. Jia et al. (2000) showed that the fixation rate of the fertilizer-NH4+ was 18%-23% at normal temperature (25 ºC) and 38%-45% at low temperature (-5 ºC to 0 ºC) under incubation condition.
In the present study we hypothesized that the climate change (temperature and CO2 elevation) may affect NH4+ fixation due to changes in soil physico-chemical and mineralogical properties. Ina clay loam soil, at Salvaterra de Magos (central Portugal), cultivated in 2012 with rice (Oryza sativa L. cv. Ariete) under intermittent flooding and conventional agronomic practices, the dynamics of pH, inorganic N and non-exchangeable NH4+ were evaluated for i) the natural ambient conditions (open-field), and ii) the temperature and CO2+temperature increase in controlled environments in field.
Material and Methods
Site description and layout of field experiment
A field experiment with a japonica rice variety (Oryza sativa L. cv. Ariete) was conducted in 2012 at Salvaterra de Magos (Tagus valley, central Portugal; latitude: 39°220.15N, longitude: 8°4425,7W, elevation: 18 m above sea level). Rice was sown on 23 May 2012 at a rate of 200 kg dry seeds ha-1 in a clay loam soil with intermittent flooding regime. The study area is the main region for rice production in Portugal. The experimental design consisted of three treatments arranged in a randomized complete block design (RCBD) with three replicates, in a total of nine blocks (Figure 1a). Each block was 4.0 m x 4.0 m, separated 4 m from each other. Treatments were as follows: elevated CO2+temperature, elevated temperature, and the unchambered control plots with the natural ambient conditions (CO2 concentration of 375±46 µmol mol-1 air, and ambient air temperature and rainfall). To change the climate variables, six large open-top chambers (OTCs) (OTC=4 m wide x 3 m height x 2 m open-top diameter, 30º tilt) covered with a polyethylene film (1 mm thickness and 75% light transmittance and provided by EstufasMinho, S.A., Fão, Portugal) except for the open-top (Figure 1b) were placed on a previous prepared (chisel and laser) lowland for paddy conditions: three OTCs for elevated CO2+temperature and three for the temperature rise by the chamber effect. Details on the construction and operation of OTCs have been provided by Pereira et al. (2013). In brief, in the three OTCs for CO2 enrichment, a system using pure industrial CO2 injection was installed to fumigate CO2 during the day-night time (24 h per day). It operated from May to October 2012 in order to have an average atmospheric CO2 concentration of 547±73 µmol mol-1 air, which represented the expected range by the middle of the 21st century.
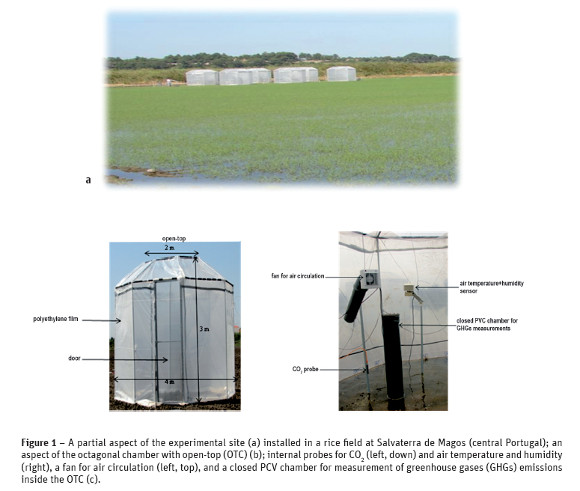
The Anthropic soil (World Reference Base for Soil Resources – FAO/ISRIC/IUSS, 2006) was representative for rice production in Portugal. It had a clay loam texture (174, 276 and 551 g kg-1 of sand, silt and clay, respectively) in the 0-60 cm layer. The dominant clay minerals in the soil were determined by the classical X-ray diffraction methods (Philips et al., 1971) and followed the order: illite (200-500 g kg-1)>smectite (20-200 g kg- 1)>vermiculite and interstratified with illite, vermiculite and smectite (<20 g kg-1). In the surface 0-20 cm layer, the bulk density was 1.1 g cm-3, pH(H2O) was 5.9, organic C was 24 g kg-1, total N was 2.4 g kg-1, and cation exchange capacity was 22.7 cmol(+) kg-1.
During the growth season, a mineral N fertilizer was split and applied twice. All the experimental plots received the same doses of fertilizers. A NP mineral fertilizer (20-20-0) was mechanically broadcast into the soil in 14 May as a basal dressing, at a rate of 60 kg NH4-N ha-1 preceding the crop seeding, and a topdressing was carried out in 10 July, at tillering, in the form of sulfamid (40% N) and at rate of 60 kg N ha-1. The basal fertilizer was incorporated into the amended soil with partially burnt crop residues (straw+root) at a depth of 20 cm, and the topdressing was applied by hand on the floodwater. No potassium was added to the soil as the soil was rich in this nutrient, whereas 60 kg of phosphorus per hectare was incorporated into the soil as part of the basal dressing.
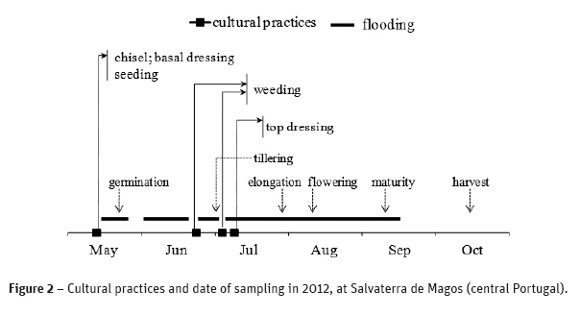
After rice seeding, the water regime was intermittent, i.e. flooding - midseason drainage (for plant rooting, one week after rice germination, and twice for weed control during a few days at tillering) - reflooding - drainage (three weeks before crop harvest) (Figure 2). Floodwater height varied from 5 cm to 20 cm during the crop season in the open-field, as well as inside the OTCs by passing through some holes at the bottom of the polyethylene film involving the OTC. Irrigation water had a pH 8.0, an electric conductivity of 0.7 dS m-1, low levels of mineral N, but a high level of chloride (71 mg Cl- l-1), and 30-48 mg Ca2+ l-1, 51-87 mg Na+ l-1, 7-10 mg K+ l-1. Ariete is a cultivar moderately sensitive to salinity and should not be negatively affected by the salts present in the water.
The experiments were kept free from weeds by using herbicides. The cultural practices used in the experiment were similar to the typical agricultural management used by Portuguese rice farmers for the last fourteen years.
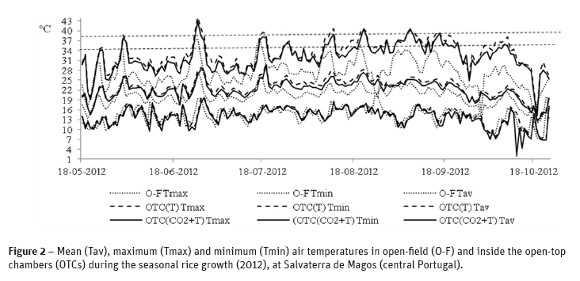
Mean air temperature during rice growth in the open-field in 2012 varied from 18 ºC in May to 21 ºC in August (Figure 3). Minimum rainfall occurred in June-July (<10 mm) and maximum in October (100 mm). The wind speed ranged from 3.8 to 8.1 m s-1 and global solar radiation presented a mean value of 5787 W m-2. Mean, maximum and minimum air temperatures inside the OTCs during the seasonal rice growth in 2012 are also shown in Figure 3. Mean air temperature variation ranged 2 ºC in May and 3 ºC in August. In summer, a higher number of days greater than 34 ºC was registered compared with the open-field, and even several days above 38 ºC (Figure 3). Inside the OTCs, solar radiation was reduced by less than 25% by the polyethylene film compared with the open-field.
Soil and floodwater sampling and analysis
Soil and floodwater samplings were taken in each plot, before and after fertilizer N application, at the end of flooding and at plant harvest (Figure 2). Soil samples were collected at 0-20, 20-40 and 40-60 cm depth, and analyzed for pH(H2O) in a 1:2.5 soil: water suspension, mineral N determined colorimetrically (Skalar), and fixed NH4+ by adaptation of the method described by Silva and Bremner (1966). Briefly, 1.0 g soil (<1.49 µm) was treated with alkaline potassium hypobromite (KOBr-KOH) solution to remove exchangeable NH4+ and soil organic N. The clear supernatant liquid was decanted and discarded, while the residue was transferred into polyethylene centrifuge tubes and washed with 0.5 M KCl to remove residual exchangeable NH4+. This operation was repeated three times. Finally, after decanting the clear supernatant liquid, the residue was treated with 5M HF-1M HCl solution and placed in an orbital shaker for 24 h to disintegrate the clay minerals containing the fixed NH4+. The non-exchangeable form released by HF-HCl was determined by spectrometry. Floodwater samples were analyzed for pH and mineral N using the above reported methods.
Statistical analysis
Soil and floodwater results were analyzed by analysis of variance (ANOVA) through the General Linear Model (GLM) using the STATISTICA 6.0 software. Means separation was performed using the Bonferronis test for p<0.05.
Results
Floodwater
In 2012, soil surface in the experimental field at Salvaterra de Magos (central Portugal) was intermittently flooded after the crop sowing till the maturity stage (three weeks before the crop harvest). Treatments (open-field, temperature and CO2+temperature elevation) did not affect the pH and mineral N in the flooded water, but the chemical properties varied significantly (p<0.001) during the growth season, especially after N fertilization (Table 1).
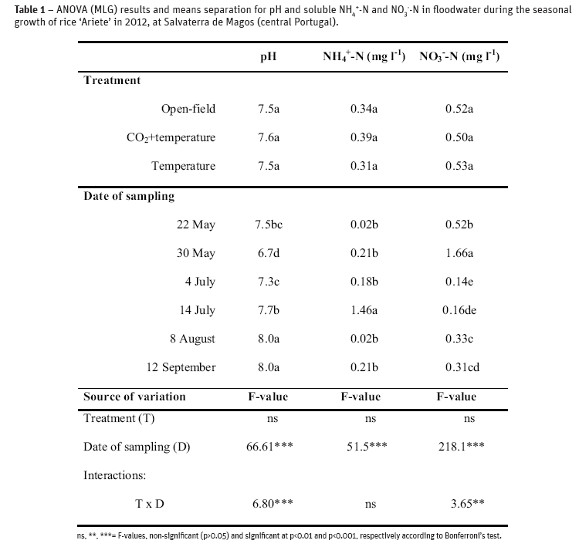
The pH in the floodwater increased during the season from pH=7.5 at initial flooding to pH=8.0 at maturity stage (Table 1). A higher NH4+ level was measured after the topdressing, whereas a high NO3- content in floodwater was determined following the basal dressing+residue incorporation into soil.
Soil
In 2012, the dynamics of some chemical soil properties was evaluated during the seasonal rice growth (Table 2). The pH(H2O) and mineral N in the medium-textured clay loam soil varied significantly during the growth cycle, but were not affected by treatments (open-field, temperature and CO2+temperature elevation). Non-exchangeable NH4+ varied significantly with treatments, date of sampling and soil depth (Table 2).
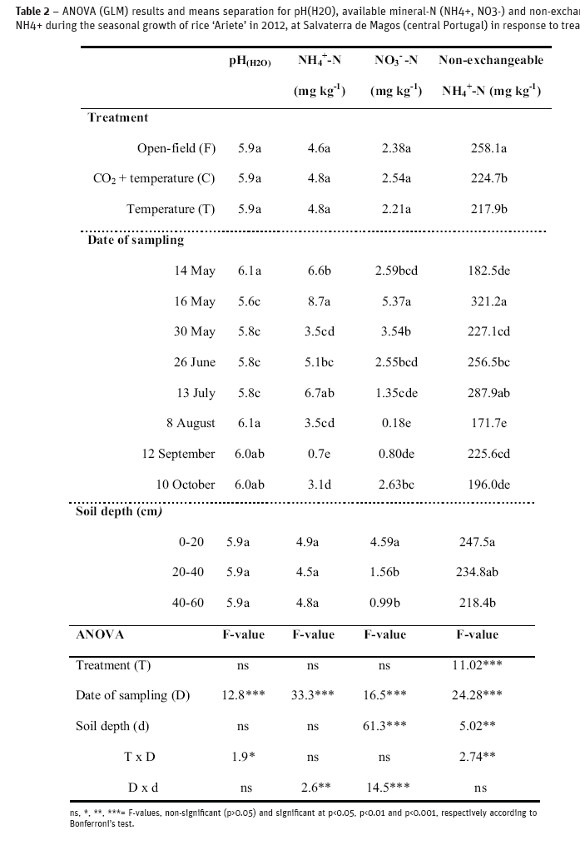
Dynamics of soil pH
Soil pH(H2O) was slightly acid at the beginning of the experiment (pH(H2O)=6.1) and decreased significantly during the season till the topdressing with sulfamid application (pH(H2O)=5.8). After the topdressing, soil pH increased again to about pH=6.0, and was maintained constant until the crop harvest (drained soil). Soil pH did not vary with soil depth (Table 2).
Dynamics of soil mineral N
Available N in soil increased significantly after the basaldressing and residue incorporation into the soil to 8.7 and 5.4 mg kg-1, respectively for NH4+ and NO3- for the mean effect of treatment and soil depth(Table 2), but decreased thereafter to the crop maturity (flooded soil) and harvest (drained soil), with insignificant levels at these stages. While NH4+ did not vary with soil depth (4.8 mg kg-1), NO3- was significantly reduced in the profile from about 5 to 1 mg kg-1.
Dynamics of non-exchangeable NH4+
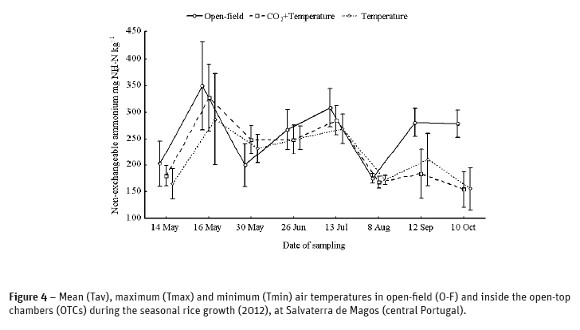
A higher concentration of non-exchangeableNH4+ (p<0.001) was measured in the open-field (258 mg NH4+-Nkg-1) for the mean effect of soil depth, compared with the OTCs, which did not vary significantly from each other (221 mg NH4+-Nkg-1). A native fixed NH4+ amounting to 183 mg kg-1 wasdetermined in the bulk soil prior to basal dressing and flooding, 28 times greater than the available NH4+ in the soil at that time. Thereafter, after the basal dressing+residue incorporation+flooding, a significant rise on fixed NH4+ was verified (321 mg NH4+-Nkg-1) and again after the topdressing to a similar amount (288 mg NH4+-Nkg-1). The level of fixed NH4+ in soil after the basal dressing corresponded to about 37 times the concentration of NH4+ in the soil solution at this time, but the proportion was 43 times greater after the topdressing. Following the topdressing, the concentration of fixed cation decreased significantly thereafter to the same level as that at the start of the experiment in the OTCs (Figure 4), but was higher (p<0.01) in the open-field (280 mg NH4+-Nkg-1).
Non-exchangeable NH4+ wassignificantly lower in the 4060 cm layer, decreasing from a mean value of 241 mg NH4+-Nkg-1 at 0-40 cm layer to 218 mg NH4+-Nkg-1 (Table 2). In the present clay loam soil, clay content did not vary significantly with soil depth (534-564 g kg-1 soil). Therefore, the higher newly fixed NH4+ in the 0-40 cm depth can be attributed to the organic residue+mineral N fertilizer incorporated at surface soil.
Discussion
Rice residues (straw+root) were partially burnt in November 2011 in the field and incorporated into the clay loam soil simultaneously with the basal mineral N, before rice sowing in 23 May 2012, followed by flooding. The incorporation of residues and flooding favored deamination of soil organic N by the facultative anaerobic microorganisms in the anaerobic conditions. Ammonium was then the predominant N form in these conditions remaining in the soil at higher levels than NO3- (Table 2). Nitrification was higher in the surface layer, in the rhizosphere (Table 2). The frequent application of 5-20 cm flood irrigation produced an almost constant anaerobic condition in lower layers, except the surface layer due to oxygenation by the water itself, the wind movement, and the roots aerenchyma (Zhang and Scherer, 2002).
Nevertheless, in our study overall available NH4+ in the soil was 28-43 times smaller than the overall fixed cation which accounted for an average of 11% of total N in the plough layer, in agreement with the literature. Just two days after flooding and application of basal N dressing, NH4+ fixation rose by 76% (Figure 4). This fact was repeated sixteen days after the N topdressing under flooding conditions with a 58% increase in relation to the bulk soil. When subtracting native fixed NH4+ from apparent fixed NH4+ after N fertilization, the net increase in fixed NH4+ was larger after the basal dressing in drained soil (+146 mg NH4+-N kg-1, corresponding to an average 322 kg N ha-1 in the 0-20 cm layer) than flooded soil after topdressing (+105 mg NH4+-N kg-1 corresponding to an average 232 kg N ha-1 in the same layer). These rates were greater than the amount of mineral N added to the soil in each growth stage (60 kg N ha-1) and it is assumed to be a result of the addition of mineral N, the higher NH4+ concentration in soil solution by mineralization of organic N compounds, and by flooding with a probable reduction of Eh (not determined) followed by an increase in the surface layer of clay minerals. Soil pH(H2O) decreased significantly (0.3 pH units) after initial flooding, indicating a Eh reduction. Especially during the first week after flooding, Zhang and Scherer (2002) also observed that the concentration of non-exchangeable NH4+ was significantly higher. Kowalenko and Cameron (1976) verified that approximately one half of the added NH4+ in the form of (NH4)2SO4 was fixed within two days after addition to the incubated clay loam waterlogged soil.
Under waterlogging and two weeks after the basal dressing or four weeks after the topdressing, the concentration of fixed NH4+ declined significantly in the our study. Zhang and Scherer (2002) observed a similar situation two weeks after fertilization, but in the contrast to the present situation where the non-exchangeable NH4+ was higher in the 0-40 cm layer, these same authors found that on the rhizosphere the fixed cation was reduced because it was released in the rhizosphere where the Eh was increased by the oxygen (O2) emitted by roots (but this diffusion decreased with distance from roots). According to Barshad (1954) and Newman (1969) the release of non-exchangeable NH4+ is favored by protons. Protons may penetrate into the wedge zones due to the somewhat expanded state of the clay minerals and displace the specifically adsorbed cations (Sparks and Liebhardt, 1982). In the present study, the reason for diffusion of fixed cation into the solution in 30 May and 8 August 2012 was not clear but apparently was related to an expanding of clay minerals and a depletion of available NH4+ in the soil solution (Table 2).
Non-exchangeable NH4+ was significantly higher in the open-field conditions compared with the climate change treatments in OTCs (Table 2). The interaction of treatments with seasonal date of sampling revealed that this difference was relevant from 8 August 2012 until harvest. Treatments for climate change in OTCs did not affect the dynamic of non-exchangeable NH4+ and other chemical properties in the soil. Maximum daily temperature in summer, from middle July to middle September 2012 was above 34 ºC in the OTCs (Figure 3), whereas in the open-field maximum temperature was below 34 ºC. This fact can explain the lower NH4+ fixation under the maximum daily temperature greater than 34 ºC inside the OTCs which agrees with some literature that refers that fixation is stronger under 30 ºC (Jia et al., 2000).
Few references on the effects of CO2 elevation on soil N dynamics are available (French et al., 2009), but on the NH4+ fixation they were not found. In the present study, the CO2 elevation simultaneous with the temperature did not affect the dynamics of non-exchangeable cation. A similar response was found for soil microbial activity (data not shown). In addition, the increase of CO2 concentration promoted photosynthesis, plant growth, and root exudates but together with the temperature elevation the crop yield was significantly reduced compared with the production under the open-field (data not shown). Further long-term field studies are recommended for the effect of global climate change on fixation of NH4+, microbial communities and dynamics of C and N in soil.
Conclusions
Non-exchangeable NH4+ was significantly higher under open-field conditions compared with the evaluated climate change scenarios of elevation of temperature with or without elevated atmospheric [CO2]. Temperature elevation reduced the fixation rate of the cation, while the CO2 concentration rise did not affect particularly the non-exchangeable form. Further studies are required for consolidation of these findings together with microbial communities and dynamics of C and N in soil.
Acknowledgements
Authors acknowledge COTArroz and its staff for facilities, climatic data, and help for field work. Authors also acknowledge the Portuguese Foundation for Science and Technology (FCT) for the financial support through projects PTDC/AGR-AAM/102529/2008 and PEst-OE/AGR/UI0245/2011, and the European Union Funds FEDER/COMPETE (Operational Competitiveness Programme). Acknowledgements are also due to Instituto Superior de Agronomia (Portugal) for clay minerals identification.
References
Barshad, I. (1954) - Cation exchange in micaceous minerals. 2. Replaceability of ammonium and potassium from vermiculite, biotite and montmorillonite. Soil Science, vol. 78, n. 1, p. 57–76. [ Links ]
Carranca, C. (1996) - Nitrogen cycling in Portuguese soils and its assessment by 15N. Doctoral Thesis (Agronomy), Lisbon, Universidade de Lisboa, 150 p. [ Links ]
FAO, ISRIC and IUSS (2006). World reference base for soil resources 2006 [em linha], Roma, Food and Agriculture Organization of the United Nations (FAO), International Soil Reference and Information Centre (ISRIC) and International Union of Soil Science (IUSS), 128 p. Disponível em: ftp://ftp.fao.org/agl/agll/docs/wsrr103e.pdf. [ Links ]
French, S.; Levy-Booth, D.; Samarajeewa, A.; Shannon, K.E.; Smith, J. and Trevors, J.T. (2009) - Elevated temperatures and carbon dioxide concentrations: effects on selected microbial activities in temperate agricultural soils. World Journal of Microbiology & Biotechnology, vol. 25, n. 11, p. 1887–1900. [ Links ]
IPCC (2007) - Summary for policy makers. In: Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M. and Miller, H.L. (Eds.) - Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA, Cambridge University Press. [ Links ]
Kowalenko, C.G. and Cameron, D.R. (1976) - Nitrogen transformations in an incubated soil as affected by combinations of moisture content and temperature and adsorption-fixation of ammonium. Canadian Journal of Soil Science, vol. 56, n. 2, p. 63-70. [ Links ]
Jia S.L.; Tang, Y.X.; Meng, C.X.. and Zhang, G.M.. (2000) - Ammonium fixation as affected by soil temperature and its management. Acta Metallurgica Sinica, vol. 6, n. 2, p. 173-178. [ Links ]
Juang, T.C. (1990) - Ammonium fixation as affected by temperature and drying-wetting effect in Taiwan soils. Proceedings of the National Science Council, Republic of China. Part B, Life Sciences, vol. 14, n. 3, p. 151-158. [ Links ]
Liu, Y.L..; Zhang, B.; Li, C.L.; Hu, F. and Velde, B. (2008) - Long-term fertilization influences on clay mineral composition and ammonium adsorption in a rice paddy soil. Soil Science Society of America Journal, vol. 72, n. 6, p. 1580-1590. [ Links ]
Lu, C.; Zhang, X.; Chen, X.; Shi, Y.; Ma, J.; Zhao, M.; Chi, G. and Huang, B. (2010) - Fixation of labeled (15NH4)2SO4 and its subsequent release in black soil of Northeast China over consecutive crop cultivation. Soil & Tillage Research, vol.106, n.2: 329–334. [ Links ]
Newman, A.C.D. (1969) - Cation exchange properties of micas. I. Relation between mica composition and potassium exchange in solutions of different pH. Journal of Soil Science, vol. 20, n. 2, p. 357–373. [ Links ]
Pereira, J.; Figueiredo, N.; Goufo, P.; Carneiro, J.; Morais, R.; Carranca, C.; Coutinho, J. and Trindade, H. (2013) - Effects of elevated temperature and atmospheric carbon dioxide concentration on the emissions of methane and nitrous oxide from Portuguese flooded rice fields. Atmospheric Environment, vol. 80, n. 1, p. 464-471. [ Links ]
Phillips, M.W.; Colville, A.A. and Ribbe, P.H. (1971) - The crystal structures of two oligoclases: A comparison with low and high albite. Zeitschrift fiir Kristallographie, vol. 133, n. 133, p. 43-65. [ Links ]
Qiu, S.-J.; Peng, P.-Q.; Li, L.; He, P.; Liu, Q.; Wu, J.-S.; Christie, P. and Ju, X.-T. (2012) - Effects of applied urea and straw on various nitrogen fractions in two Chinese paddy soils with differing clay mineralogy. Biology & Fertility of Soils, vol. 48, n. 2, p. 161-172. [ Links ]
Scherer, H.W. and Zhang, Y.S. (1999) - Studies on the mechanisms of fixation and release of ammonium in paddy soils after flooding. I. Effect of iron oxides on ammonium fixation in paddy soils. Journal of Plant Nutrition and Soil Science, vol. 162, n. 6, p. 593–597. [ Links ]
Scherer, H.W. and Zhang, Y.S. (2002) - Mechanisms of fixation and release of ammonium in paddy soils after flooding. III Effect of the oxidation state of octahedral Fe on ammonium fixation. Journal of Plant Nutrition and Soil Science, vol. 165, n. 2, p. 185–189. [ Links ]
Schneiders, M. and Scherer, H.W. (1998) - Fixation and release of ammonium in flooded rice soils as affected by redox potential. European Journal of Agronomy, vol. 8, n. 3-4, p. 181-189. [ Links ]
Shi, D.; Xu, Y.; Hopkinson, B.M. and Morel, F.M.M. (2010) - Effect of ocean acidification on iron availability to marine phytoplankton. Science, vol. 327, n. 5966, p. 676-679. [ Links ]
Silva, J.A. and Bremner, J.M. (1966) -. Determination and isotope-ratio analysis of different forms of nitrogen in soils: 5. Fixed ammonium. Soil Science Society of American Proceedings, vol. 30, n. 5, p. 587–594. [ Links ]
Sparks, D.L. and Liebhardt, W.C. (1982) - Temperature effects on potassium exchange and selectivity in Delaware soils. Soil Science, vol. 133, n. 1, p. 10–17. [ Links ]
Zhang, Y.S. and Scherer, H.W. (2002) -Mechanisms of fixation and release of ammonium in paddy soils after flooding. IV. Significance of oxygen secretion from rice roots on the availability of non-exchangeable ammonium – a model experiment. Biology & Fertility of Soils, vol. 35, n. 3, p. 184–188. [ Links ]
Zhang, Y.; Liao, J.; Li, F.; Huang, Y.; Hu, R. and Yuan, Z. (2002) - Fixed ammonium content of chief paddy soil types in Hunan Province and its influencing factors. Ying Yong Sheng Tai Xue Bao, vol. 13, n. 6, p. 693-697. [ Links ]
Recebido/Received: 2013.09.28
Aceitação/Accepted: 2013.10.29
Notes
* Centro de Engenharia dos Biossistemas (CEER), Instituto Superior de Agronomia (ISA), Universidade de Lisboa (UL), 1349-017 Lisboa, Portugal.














