Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.37 no.4 Lisboa dez. 2014
ARTIGO
Sanguisorba hybrida: pharmacognostic studies and antimicrobial activity evaluation of crude extracts
Sanguisorba hybrida: estudo farmacognóstico e avaliação da atividade antimicrobiana de extratos brutos
Ana Margarida Madureira1, Aida Duarte1, Ana Isabel Correia2, Generosa Teixeira3
1 Universidade de Lisboa, Faculdade de Farmácia de Lisboa, iMed Universidade de Lisboa, Avenida Prof. Gama Pinto, 1649-003, Lisboa, Portugal. E-mails: afernand@ff.ulisboa.pt; aduarte@ff.ulisboa.pt
2 Universidade de Lisboa, Faculdade de Ciências de Lisboa, Centro de Biologia Ambiental - Ce3C, C2, Campo Grande, 1749-016, Lisboa, Portugal. E-mail: aicorreia@fc.ulisboa.pt
3 Universidade de Lisboa, Faculdade de Farmácia de Lisboa, Centro de Biologia Ambiental - Ce3C, Avenida Prof. Gama Pinto, 1649-003, Lisboa, Portugal. E-mail: gteixeira@ff.ulisboa.pt, author for correspondence
ABSTRACT
The genus Sanguisorba L. (Rosaceae) includes perennials that are distributed throughout the northern hemisphere. Some species of this genus are known by their medicinal interest. In the present work the pharmacognostic characterization and the potential antimicrobial activity of several extracts of Sanguisorba hybrida, endemic in Portugal, were evaluated. The leaves of the plant have been observed under microscopy techniques, tector and secretor multicellular trichomes were seen on both leaf surfaces. With histochemical tests the in situ localization of compounds was recognized and the most relevant were mucilages, phenols and terpenoids. Powdered plant material was extracted with n-hexane, dichloromethane, ethyl acetate, methanol and water. Their phytochemical survey, through TLC on silica gel plates and the proper reagents, was performed and the previous results with histochemical tests were confirmed. All extracts were tested against reference and multiresistant bacterial strains: Gram-positive (Enteroccocus faecalis, Staphylococcus aureus, Staphylococcus epidermidis and Mycobacterium smegmatis); Gram-negative (Pseudomonas aeruginosa, Salmonella typhimurium, and Klebsiella pneumoniae) and the yeast Candida albicans. The minimum inhibitory concentrations (MIC) were determined by the serial broth micro dilution method. Appropriated antibiotics were used as controls. The methanol and water extracts showed better antimicrobial activity than n-hexane, dichloromethane, ethyl acetate extracts. Gram-positive bacteria were the most sensitive and the MIC values of 3.50-1.75 µg/mL were obtained using those polar extracts against Staphylococcus aureus, including strains resistant to meticillin (MRSA).
Palavras-chave: Sanguisorba hybrida, Rosaceae, pharmacognostic studies, antimicrobial activity
RESUMO
O género Sanguisorba L. (Rosaceae) engloba plantas perenes distribuídas por todo o hemisfério norte, sendo algumas espécies conhecidas como medicinais. Neste trabalho realizou-se o estudo farmacognóstico e avaliou-se a atividade antimicrobiológica de vários extratos de uma espécie deste género, endémica de Portugal, Sanguisorba hybrida. Recorrendo a diferentes técnicas de microscopia observou-se, nas duas epidermes foliares, tricomas multicelulares de dois tipos, tectores e secretores. Com diferentes testes histoquímicos procedeu-se à localização in situ dos principais grupos de compostos. Os mais representativos foram mucilagens, fenóis e terpenóides. No estudo fitoquímico o material vegetal pulverizado foi extraído sequencialmente com solventes de polaridades crescentes: n-hexano (n-hex), diclorometano (CH2Cl2), acetato de etilo (AcOEt), metanol (MeOH) e água (H2O). Foi determinado o respetivo perfil fitoquímico através de cromatografia em placas de sílica. Todos os extratos foram posteriormente testados contra estirpes de bactérias sensíveis e multirresistentes Gram-positivas (Enteroccocus faecalis, Staphylococcus aureus, Staphylococcus epidermidis and Mycobacterium smegmatis), Gram-negativas (Pseudomonas aeruginosa, Salmonella typhimurium e Klebsiella pneumoniae) euma levedura, a Candida albicans. A concentração mínima inibitória (MCI) foi determinada pelo método de diluição em meio líquido. Foram usados antibióticos e antifúngicos como controlos. Os extratos polares, metanólico e aquoso, apresentaram atividade antimicrobiana superior à dos restantes extratos, menos polares. As bactérias Gram-positivas, Staphylococcus aureus, incluindo as estirpes resistentes à meticilina (MRSA) ensaiadas, foram as mais sensíveis aos referidos extratos (MCI 3,50-1,75 µg/mL).
Keywords: atividade antimicrobiana, estudo farmacognóstico, Rosaceae, Sanguisorba hybrida
Introduction
Plants are major sources of bioactive molecules, other health formulations and supplements used in the treatment and prevention of diseases. Currently it appears that infectious diseases are a serious problem due to the rapid emergence of new infections and to the growing drug-resistance to the antibiotics commonly used in therapy and therefore there is a great need to find new and more efficient antimicrobial agents (Sánchez-Medina et al., 2001; Weckesser et al., 2007). The genus Sanguisorba L. belongs to the Rosaceae family and to the Rosoideae subfamily. It includes perennials that are distributed throughout the Mediterranean basin. Among their species we can highlight Sanguisorba officinallis L. and Sanguisorba minor Scop. with medicinal interest documented: hypoglycemic and hemostatic properties (Reher et al., 1991; Goun, 2002), antipyretic (Camejo-Rodrigues et al., 2003), antimicrobial (Sardari et al.,1998; Kokoska, 2002) and antiviral activities (Kim, 2001; Abad et al., 2000; Bedoya et al., 2001), hypoallergenic (Park, 2004), and the effectiveness in the treatment of gastric ulcers in mice (Gurbuz et al., 2005).
Sanguisorba hybrida (L.) Nordborg is an endemic plant species in Portugal, distributed mainly in the South Central region (Navarro and Garmendia, 1998), common known as agrimónia-bastarda, agrimónia-brava and pimpinela-híbrida. The specific epithet hybrida is allegedly the fact that this species is morphologically between S. officinalis and S. minor. To the best of our knowledge no studies about the micromorphology, the phytochemistry and the antimicrobial activity of S. hybrida have yet been reported. The main goal of this study was to achieve the pharmacognostic characterization of S. hybrida leaves, including their micromorphological description, their preliminary phytochemical study and evaluation of the potential of n-hexane, dichloromethane, ethylacetate, methanol and water extracts against human pathogens.
Materials and Methods
Plant material
Small branches of S. hybrida were collected in SW Portugal (38º 8 N - 8º 33 W), during June and July of 2009 and 2010. Plant material was identified at the Lisbon Botanical Garden Herbarium (LISU 221369).
Light microscopy Samples of fresh and fixed material were used. Fixation followed the usual procedures in a 2.5% glutaraldehyde solution (Hayat, 1981). Observations were made on a stereomicroscope Nikon SMZ-2T and on a microscope Nikon Labophot 2. Several histochemical tests to detect the main chemical groups were made, according to previous works (Rodrigues et al., 2013; Rodrigues et al., 2008; Teixeira et al., 2013). Autofluorescence and induced fluorescence with fluorochromes for flavonoid detection (Charrière-Ladreix, 1973) were done on a microscope Olympus BX51 epifluorescence, equipped with a camera model DP50 and the software Studio Lite, 1.0.124 and results were semi-quantitatively analyzed, between negative (-) and strongly positive (+++).
Scanning Electron Microscopy
The plant material preparation followed the usual procedures (Hayat, 1981; (Rodrigues et al., 2013; Rodrigues et al., 2008; Teixeira et al., 2013). Observations were done with a Jeol JSM - T220 scanning microscope at 15 kV.
Preparation of Plant Extracts
The plant material was dried in the dark, at room temperature and powdered. Approximately 100g of powder was extracted sequentially, with increasing polarity solvents [n-hexane (Hex), dichloromethane (CH2Cl2), ethyl acetate (AcOEt), methanol (MeOH) and water (H2O)], for 24 h, at room temperature with occasional shaking. The extracts obtained were filtered; evaporated under reduced pressure and stored at 4 ºC until use, with the exception of the aqueous extract which has been freeze-dried.
Phytochemical Screening
The extracts were dissolved in proper solvents, applied on silica gel thin layer chromatography plates and de¬veloped with appropriate mixtures of eluents. The plates, containing an application of each extract (Hex, CH2Cl2, AcOEt, MeOH e H2O), were revealed with spray specific reagents for each class of substances (Wagner and Blader, 1996). Results were displayed semi-quantitative in a range between absence (-) and strongly present (+++).
Screening for Antimicrobial Activity Microbial Strains
All extracts were in vitro tested against sensitive and multiresistant bacterial strains: Gram-positive, Enteroccocus faecalis ATCC 51299, Staphylococcus au-reus ATCC 6538, ATCC 43866 (Methicillin-resistant S. aureus; MRSA), ATCC 700699 (Vancomycin intermediated S. aureus; VISA) and ATCC 106760 (VISA), Staphylococcus epidermidis ATCC 35984; Gram-negative, Pseudomonas aeruginosa ATCC 9027, Salmonella typhimurium ATCC 13311 and Klebsiella pneumonia ATCC 9997). It was also assessed the sensitivity of the acid-alcohol resistant bacillus Mycobacterium smegmatis CIP 607 and of the yeast Candida albicans ATCC 10231. The test microorganisms were obtained from the culture collection of Prof. Aida Duarte, Faculty of Pharmacy, Lisbon University.
Determination of the minimal inhibitory concentration (MIC)
The MIC was determined by the serial broth micro dilution method (Cos et al., 2006; CLSI, 2008). Tests were performed in Mueller–Hinton broth medium, in 96-well micro plates, as follows: to 100 µL of Mueller-Hinton medium, 100 µL of each extract solution to be tested, were added. The extracts of S. hybrida were tested in concentrations ranging between 500-1.75 µg/mL. An innocuous of each microorganism was added (10 µL; final concentration 104 cfu/ mL). The last row, containing the microorganisms in Mueller–Hinton medium without the test sample, was used as a bacteria control. Appropriated antibiotics were used as reference for antibacterial activities and all the assays were repeated at least by three independent experiments. The micro plates were covered and incubated at 37 °C for 24-h, with the exception of bacterial strains E. faecalis and M. smegmatis who required incubation periods of 48-h. The microbial development was evaluated by measuring the absorbance of the micro plates, at the wavelength of 630 nm, in a micro plate reader Biotek ELX808. It corresponds to the lowest concentration of an extract that inhibits the development of a certain microorganism. Positive values were considered when MIC < 100 µg/mL (Cos et al., 2006).
Results
Micromorphological Study
S. hybrida leaves show epidermal cells with similar characteristics on both leaf surfaces: irregular cells with seen on the abaxial surface, distributed randomly, without specific guidance along the leaf area (Fig. 1). The stomatal index was estimated at 21.4. On the upper and lower epidermal surfaces secretory and tector trichomes are seen with equal structural features but with different abundance and distribution: in the adaxial surface the tector trichomes, with 3-6 large and thin cells, are few; the secretory trichomes, with a minimum of 5 cells in the neck and 2-3 cells in the head (Fig. 2), are abundant throughout the leaf blade. In the abaxial surface it is the opposite, greater abundance of tector trichomes, scattered all over the leaf blade and lower abundance of secretory trichomes, which are mostly over the leaf main vein (Fig. 3).
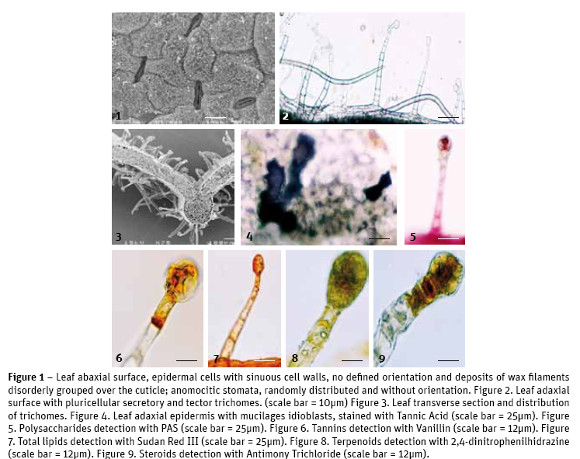
In the analysis of leaf cross-sections we gather information about its anatomy. The average total thickness of leaves was 127.7 ± 22.7 µm. Upper epidermal cells are covered by a cuticle with average thickness of 3.4 ± 1.3 µm. The mesophyll is typical of dicotyledon plants, with an asymmetrical chlorophyll parenchyma: the palisade parenchyma with 1-2 layers of elongated cells arranged perpendicularly to the leaf surface, without intercellular spaces and the spongy parenchyma with 3-4 layers of rounded cells, showing small intercellular spaces. The two parenchyma tissues, palisade and spongy, showed an average thickness of 35.4 ± 6.5 µm and 55.2 ± 8.3 µm, respectively. It was also made the visualization of calcium oxalate crystals, mainly druse type but also some raphids, in idioblasts nearby the vessels.
Histochemical Study
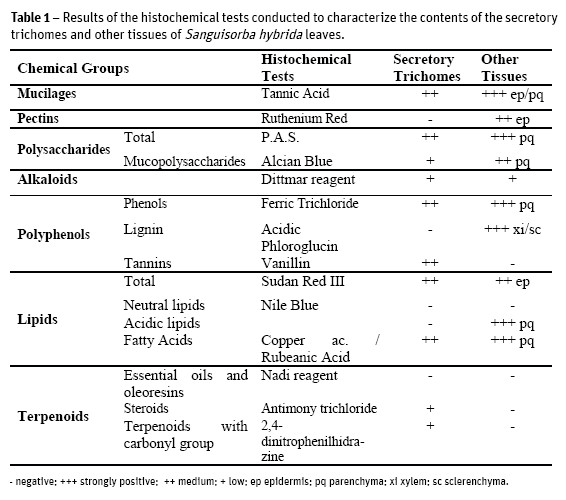
Several histochemical tests were conducted to characterize the contents of the different tissues of S. hybrid leaves. The results are briefly reported on Table 1.
The use of Tannic Acid for mucilages detection, revealed positive in tector and secretory trichomes and also in epidermal (Fig. 4) and parenchyma idioblasts.
The application of Ruthenium Red showed that only the cell walls of the epidermal cells have pectins, for the appearance of the pink color. The use of PAS for total polysaccharides revealed pinkness in the head cells of secretory trichomes (Fig. 5) and in parenchyma cells. A similar localization was seen with Alcian Blue for acidic polysaccharides.
Dittmar reagent for alkaloids stained positively, light brown color, the parenchyma tissue and the heads of secretory trichomes. When applying Ferric Trichloride, for phenols, the mesophyll stained black brownish as well as the tector trichomes while the secretory trichomes presented a light brown color only in their head cells. The treatment of leaf sections with Vanillin, for tannins, has just revealed orange brown coloring in the head cells of secretory trichomes (Fig. 6). As expected, acidic Phloroglucin detected lignin in xylem conductive vessels and in sclerenchyma cells. The application of Sudan Red III, for total lipids detection, revealed the orange coloration on the head of secretory trichomes and epidermal cell walls (Fig. 7). The Nile Blue test for neutral lipids was negative but positive for acidic lipids, in the basal cells of secretory trichomes, negative for their cell's head and positive as well for parenchyma cells. With Copper acetate / Rubeanic Acid for fatty acids, all structures with the exception of tector trichomes stained dark green.
No essential oils and oleoresins were detected with the use of Nadi reagent. Other terpenoids, with 2,4-dinitrophenilhidrazine (Fig. 8), and steroids, with Antimony Trichloride (Fig. 9), were slightly detected as yellow-orange colored only on the secretory trichomes head. Under fluorescence microscopy the presence of flavonoids was confirmed.
Phytochemical screening
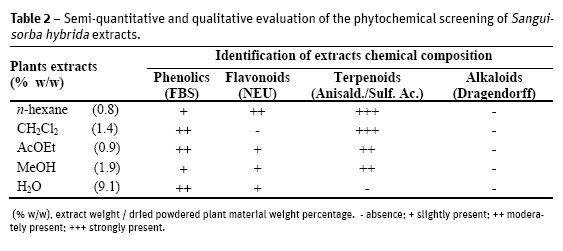
The semi-quantitative and qualitative results of the phytochemical screening, as well as the yield of the dried extracts (as w/w percentage) of the starting dried material) are presented on Table 2. All extracts were rich in terpenoids except the water extract. Phenolics and flavonoids were also present in all extracts but not in the dichloromethane. It is clear that all plant extracts were negative to alkaloids.
Evaluation of minimal inhibitory concentration (MIC)
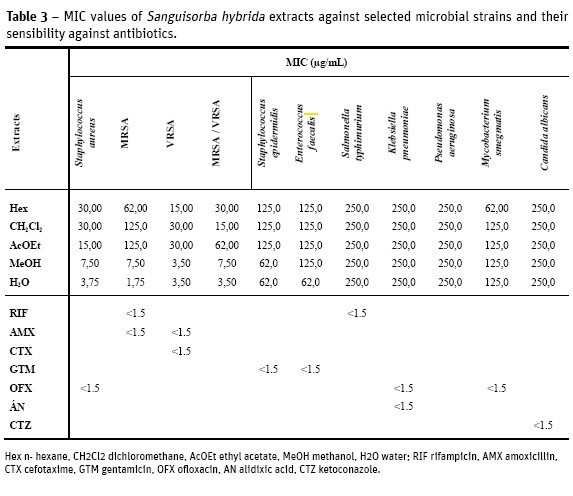
The MIC of S. hybrida extracts against selected human pathogens was evaluated and results are displayed on Table 3. Among all the tested extracts, the methanol and water extracts presented higher activity than n-hexane, dichloromethane and ethyl acetate extracts. The polar extracts strongly inhibit the growth of Staphylococcus aureus strains including those resistant to meticillin (MRSA) and vancomicin (VISA) with MIC between 3.50-1.75 µg/mL. Those extracts also exhibited moderate activity (62.0 µg/ mL) against S. epidermidis. The non polar n-hexane extract was active (62.0 µg/mL) against the acid-alcohol resistant bacillus Mycobacterium smegmatis.
No activity was observed when Gram-negative and fungi were assayed.
Discussion
The pharmacognostic study of S. hybrida using different techniques allowed a better knowledge of this species and from their characteristics it can be inferred that this plant is well adapted to its ecosystem.
We found hipostomatic leaves, which contributes to reduce water loss by transpiration and therefore prevent dehydration (Wilkinson, 1979; Mott et al., 1982); Lleras, 1997). The stomata distribution and the dorsiventral mesophyll are characteristic of plants that grow in mesophytic environments, that is, plants that are adapted to a moderately moist environment. The presence of wax deposits on the epidermis surface is essential both in the maintenance of temperature and in the water retention on the leaf sheet, once again contributing to an efficient plant hydration (Wilkinson, 1979). The microscopic observations showed the existence of a considerable number of calcium oxalate crystals, mainly concentrated in the region of the main vessels. These might be involved in the photosynthetic rate improvement, allowing a better light distribution through the mesophyll, but also providing mechanical support and plant protection against predators (Metcalfe and Chalk, 1988). The existence of trichomes in both leaf surfaces works similarly, in the protection against biotic and abiotic elements (Fahn, 1986; Fahn and Cutler, 1992; Larcher, 2000). In the histochemical study (Table 1) there were detected mucilages, pectins, total and acidic polysaccharides. All these chemical groups contribute to maintain cellular hydration (Meyberg, 1988; Fahn, 1979) and some authors suggest that they also reduce transpiration (Gregory and Baas, 1989) which is conforming to the environmental needs of this plant, as well as with characteristics common to other Rosaceae (Evans, 2009).
All leaf tissues showed a diverse chemical composition, including some types of terpenoids and polyphenols (Table 1), also confirmed in the phytochemical screening of S. hybrida extracts (Table 2).
The terpenoids have been described as one of the groups of compounds correlated to the pharmacological activity of S. officinalis and S. minor (Goun et al., 2002; Li et al., 1992; Viano et al., 1999), so its detection in S. hybrida seems to corroborate the importance of these compounds in the genus Sanguisorba and can be a very promising sign for possible pharmacological activities in this taxa. The presence of tannins is also associated to the properties attributed to S. officinalis and S. minor (Goun et al., 2002; Li et al., 1992; Viano et al., 1999) and, therefore, of the utmost relevance.
The alkaloids have been detected in small concentration, through Dittmar reagent, in the head cells of secretory trichomes and in the parenchyma tissue (Table 1). In plants the existence of this chemical group is often associated with the protection against herbivores. However in the phytochemical screening of S. hybrida extracts (Table 2) its presence was not detected. A possible explanation for the histochemical result may be on the chemical composition of Dittmar reagent, with iodine compounds, which can react with starch and also with some proteins (Evans, 2009). The antimicrobial activity was performed by the serial broth microdilution method. Several microorganisms were assayed namely several Staphylococcus aureus strains including a bacterial biofilm model (ATCC 6538) due to their higher resistance against antimicrobial agents. Vancomicin and meticillin resistant strains were also included, as well as S. epidermidis and Enterococcus faecalis. Gram-negative bacteria enclosed Salmonella typhimurium (Entero-bacteriacea non encapsulated) Klebsiella pneumoniae (Enterobacteriacea encapsulated and Pseudomona aeruginosa (non-Enterobacteriacea). The acid-alcohol resistant bacillus Mycobacterium smegmatis was also tested, as a fast-growing model for anti-mycobacterium preliminary assessment of the extracts activity (Cowan and Talaro, 2009). To evaluate the antifungal activity C. albicans was the selected microorganism (Cos et al., 2006).
The bioactivity of the extracts was heterogeneous, when comparing Gram-positive and Gram-negative bacteria, probably due to the differences in their cell walls composition and structure, less complex in Gram-positive (Cos et al., 2006; Slama, 2008). From the results presented in Table 3 it should be highlighted that S. hybrida polar extracts displayed notable antibacterial activity (MIC 7.50-1.75 µg/mL) against all the S. aureus strains assayed (biofilms, MRSA and VISA).
Correlating those results with the chemical composition of the extracts (Table 2), it can be inferred that the activity might be related to their rich terpenoid and phenolic contents. The bioactivity of phenols (Nikitina et al., 2007; Mason and Wasserman, 1987), tannins (Scalbert, 1991; Jones et al., 1994) terpenoids (Rodrigues et al., 2012; Scortichini and Rossi, 1991), flavonoids and flavonoid glycosides (Ghasemzadeh and Ghasemzadeh, 2011; Tsuchiya et al., 1996) against a wide range of microbes has been referenced. The secondary metabolites are known to be produced by plants in response to injuries and its not be surprising that they have been found to be effective against a wide array of human pathogens in vitro. Their activity is probably due to their ability to complex with extracellular and soluble proteins and to complex with bacterial cell walls. More lipophilic flavonoids may also disrupt microbial membranes (Tsuchiya et al., 1996). Condensed tannins have also been determined to bind cell walls of ruminal bacteria (Jones et al., 1994).
Conclusions
The present study contributed to the pharmacognostic characterization of S. hybrida and demonstrated its potential as a natural antimicrobial agent.
The leaves tissues, including secretory trichomes, showed positive with most of the histochemical tests aimed at the main chemical groups. The extracts phytochemical screening confirmed the presence of terpenoids and polyphenols in most of them. The active chemical compounds of the methanol and water extracts showed better antimicrobial activity than those found in the n-hexane, dichloromethane and ethyl acetate extracts. Gram-positive bacteria were the most sensitive and the MIC values 7.50-1.75 µg/mL were obtained using those polar extracts against Staphylococcus aureus, including biofilms, MRSA, VISA and strains.
Acknowledgements
The authors gratefully acknowledge the technical support from Telmo Nunes in capturing SEM images.
References
Abad, M.J. Bermejo, P., Sanchez Palomino, S., Chiriboga, X. and Carrasco, L. (2000) - Search for antiviral activity in higher plant extracts. Phytotherapy Research, vol.14, n. 8, p. 604-607. [ Links ]
Appelbaum, P.C. (2007) - Microbiology of antibiotic resistance in Staphylococcus aureus. Clinical Infectious Diseases vol. 45, (Supplement 3), p. S165-S170. [ Links ]
Bedoya, L.M., Sanchez- Palomino S., Abad M.J., Bermejo P. and Alcami, J. (2001) - Anti-HIV activity of medicinal plant extracts. Journal of Ethnopharmacology, vol. 77, n. 1, p. 113-116. [ Links ]
Camejo-Rodrigues J., Ascensão, L., Bonet M.À. and Vallès J. (2003) - An ethnobotanical study of medicinal and aromatic plants in the Natural Park of Serra de São Mamede (Portugal). Journal of Ethnopharmacology, vol. 89, n. 2, p. 199-209. [ Links ]
Charrière-Ladreix, Y. (1973) - Étude de la sécrétion flavonoidique des bourgeons de Populus nigra
L. var., italica. Cinétique du phénomène glandulaire, ultrastructure et évolution du tissu glandulaire, Journal de Microscopie, vol. 17, p. 299-316.
CLSIClinical Laboratory Standards Institute. (2008) - Performance Standards for Antimicrobial Susceptibility Testing: 18th Informational Supplement. Clinical and Laboratory Standards Institute, Wayne. [ Links ]
Cos, P., Vlietinck A.J, Berghe D.V, Maes L. (2006) -Anti-infective potential of natural products: How to develop a stronger in vitro proof-of-concept. Journal of Ethnopharmacology, vol. 106, n.3, p.290-309. [ Links ]
Cowan, M.K. and Talaro, K.P. (2009) - Microbiology: A Systems Approach. McGraw-Hill International Edition. [ Links ] Evans, W.C. (2009) - Trease and Evans Pharmacognosy. 16th Edition. Saunders Company Ltd. London [ Links ]
Fahn, A. (1979) - Secretory tissues in vascular plants. London, Academic Press. [ Links ]
Fahn, A. (1986) - Structural and functional properties of trichomes of xeromorphic leaves. Annals of Botany, vol. 57, n. 5, p. 631-637. [ Links ]
Fahn, A and Cutler, D. (1992) - Xerophytes. Encyclopedia of Plant Anatomy vol.8, Ed. Gebruder Borntreager, Berlim, p.176 [ Links ]
Ghasemzadeh, A. and Ghasemzadeh, N. (2011) -Flavonoids and phenolic acids: Role and biochemical activity in plants and human. Journal of Medicinal Plants Research, vol. 5, n. 31, p. 6697-6703. [ Links ]
Goun, E.A., Petrichenko, V. and Solodnikov, S. (2002) - Anticancer and antithrombin activity of Russian plants. Journal of Ethnopharmacology, vol. 81, n. 3, p. 337-342 [ Links ]
Gregory, M. and Baas, P. (1989) -Mucilage cells in dicotyledons. Israel Journal of Botany, vol. 30, p. 125-174. [ Links ]
Gurbuz, I. (2005) - Anti-ulcerogenic activity of some plants used in folk medicine of Pinarbasi (Kayseri, Turkey). Journal of Ethnopharmacology, vol. 101, n. 1, p. 313-318. [ Links ]
Hayat, M. (1981) - Principles and techniques of electron microscopy. Biological Applications. 2nd ed. London. Ed. Arnold Publ. [ Links ]
Jones, G.A., Mc Allister, T.A., Muir, A.D. and Cheng, K.J. (1994) -Effects of Sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Applied Environmental Microbiology, vol. 60, n. 4, p. 1374-1378. [ Links ]
Kim, T.G., Kang, S. and Jung, K. (2001) - Antiviral activities of extracts isolated from Terminalis chebula Retz., Sanguisorba officinalis L., Rubus coreanus Miq. and Rheum palmatum L. against Hepatitis B Virus. Phytotherapy Research, vol. 15, n. 8, p. 718-720. [ Links ]
Kokoska, L., Polesny Z., Rada V., Nepovim A. and Vanek T. (2002) - Screening of some Siberian medicinal plants for antimicrobial activity. Journal of Ethnopharmacology, vol. 82, n. 1, p. 51-53. [ Links ]
Larcher, W. (2004) - Ecofisiologia vegetal. Ed. Rima Artes e Textos, São Carlos, Brasil p.50-53. [ Links ]
Li, G.X. (1992) -Pharmacology, Toxicity and Clinic of Traditional Chinese Medicine. Tianjin Science and Technique Translation Publishing House, Tianjin, p. 207-208. [ Links ]
Lleras, E. (1997) - Differences in stomatal number per unit area within the same species under different micro-environmental conditions: a working hypothesis. Acta Amazônica, vol. 7, n. 4, p. 473-476. [ Links ]
Mason T.L. and Wasserman B.P. (1987) -Inactivation of Red Beet Betaglucan Synthase by Native and Oxidized Phenolic Compounds. Phytochemistry, 26: 2197 -2202. [ Links ]
Metcalfe, C.R. and Chalk, L. (1988) - Anatomy of Dicotyledons. Volume1. 2nd ed. Clarendon Press, Oxford Sciences Publication, Oxford, p. 63-66. [ Links ]
Meyberg, M. (1988) -Cytochemistry and ultrastructure of the mucilage secreting trichomes of Nymphoides peltata (Menyanthaceae). Annals of Botany, 6vol. 62, p. 537-548. [ Links ]
Mott, K.A., Gibson, A.C. & O'Leary, J.W. (1982) - The adaptative significance of amphistomatic leaves. Plant Cell Environment, vol. 5, n. 6, p. 455-460. [ Links ]
Navarro, C., Garmendia, F. (1998) - Flora Iberica, Sanguisorba. Vol. VI. Real Jardín Botánico, CSIC. Madrid. [ Links ]
Nikitina, V., Kuzmina, L. Yu. Melentev, A.I. and Shendel, G.V. (2007) -Antibacterial Activity of Polyphenolic Compounds Isolated from Plants of Geraniaceae and Rosaceae Families. Applied Biochemistry and Microbiology, vol. 43, n. 6, p. 629634 [ Links ]
Park, K., Koh D, Kim K, Park J. and Lim, Y. (2004) -Antiallergic Activity of a Disaccharide isolated from Sanguisorba officinalis. Phytotherapy Research, vol. 18, n. 8, p. 658-662. [ Links ]
Parkhurst, D.F. (1978) - The adaptative significance of stomata occurrence on one or both surfaces of leaves. Journal of Ecology, vol. 66, p. 367-383. [ Links ]
Reher, G., Slijepcevic, M. and Krans, L. (1991) - Hypoglycemic activity of triterpenes and tannins from Sarcopoterium spinosum and two Sanguisorba species. Planta Medica. vol. 57, n. S2, p. 57-58 [ Links ]
Rodrigues, L., Póvoa, O., Teixeira, G., Figueiredo, A.C., Moldão, M. & Monteiro, A. (2013) - Trichomes micromorphology and essential oil variation at different developmental stages of cultivated and wild growing Mentha pulegium L. populations from Portugal. Industrial Crops and Products, vol. 43, n. 1, 692-700. [ Links ]
Rodrigues, L., Duarte, A., Figueiredo, A.C., Brito, L., Teixeira, G., Moldão, M. & Monteiro, A. (2012) - Chemical composition and antibacterial activity of the essential oils from the medicinal plant Mentha cervina L. grown in Portugal. Medicinal Chemical Research, vol. 21, n. 11, p. 3485-3490. [ Links ]
Rodrigues, L., Monteiro, P., Póvoa, O., Teixeira, G., Moldão, M., Figueiredo, A.C. & Monteiro, A. (2008) - Morphology of secretory structures and essential oil composition in Mentha cervina L. from Portugal. Flavour and Fragrance Journal, vol. 23, n. 5, p. 340-347 [ Links ]
Sánchez-Medina, A., García-Sosa, K., May-Pat, F., Pena-Rodríguez1, L.M. (2001) - Evaluation of biological activity of crude extracts from plants used in Yucatecan Traditional Medicine Part I. Antioxidant, antimicrobial and glucosidase inhibition activities. Phytomedicine, vol. 8, n. 2, p. 144-151. [ Links ]
Sardari, S. Gholamreza A., Ronald G. and Mohsen D. (1998) - Phytopharmaceuticals. Part 1. Antifungal activity of selected Iranian and Canadian plants. Pharmaceutical Biology, vol. 36, n. 3, p. 180-188. [ Links ]
Scalbert, A. (1991) - Antimicrobial Properties of Tannins. Phytochemistry, vol.30, p.3875-3883 [ Links ]
Scortichini, M. and Rossi, M.P. (1991) -Preliminary in vitro evaluation of the antimicrobial activity of terpens and terpenoids towards Erwinia amylovora (Burrill). Jounal of Applied Bacterialogy, vol. 71, n. 2, p. 109-112. [ Links ]
Slama, T. (2008) - Gram-negative antibiotic resistance: there is a price to pay. Critical Care, 12 (Suppl. 4): 4. [ Links ]
Teixeira, G., Correia, A.I., Vasconcelos, T., Feijão, D. and Madureira, A.M. (2013) - Lavandula stoechas subsp. luisieri and L. pedunculata - phytochemical study, micromorphology and histochemistry. Re-vista de Ciências Agrárias, vol. 36, n. 2, p. 220-228. [ Links ]
Tsuchiya, H., Sato, M., Miyazaki, T., Fujiwara, S., Tani-gaki, S., Ohyama, M., Tanaka, T. and Linuma, M. (1996) - Comparative Study on the Antibacterial Activity of Phytochemical Flavanones against Methicillin-Resistant Staphylococcus aureus, Journal of Ethnopharmacology, vol. 50, n. 1, p. 27-34. [ Links ]
Viano, J., Masotti, E. and Gaydou, E. (1999) - Nutritional Value of Mediterranean Sheeps Burnet (Sangisorba minor Ssp. muricata). Journal of Agriculture Food Chemistry, vol. 47, n. 11, p. 4645-4648. [ Links ]
Yasunaka, K., Abe, F., Nagayama, A., Okabe, H., Lozada-Pérez, L., López-Villafranco, E., Mu niz, E.E., Aguilar, A., Reyes-Chilpa, R. (2005) -Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. Journal of Ethnopharmacology, vol. 97, n. 2, p. 293-299. [ Links ]
Wagner H, Blader S. (1996) - Plant Drug Analysis. 2nd ed. Springer-Verlag. Berlin. [ Links ]
Weckesser, S., Engel, K., Simon-Haarhaus, B., Witt-mer, A., Pelz, K., Schempp, C.M. (2007) -Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine, vol. 14, n. 7-8, p. 508-516. [ Links ]
Wilkinson, H.P. (1979) -The Plant Surface (Mainly Leaf). Part I: Stomata. In Metcalf, C.R. and Chalk, L. Anatomy of Dicotyledons, Vol I. Ed. Claredon Press, Oxford, p. 98-117 [ Links ]
Recebido/Received: 2014.07.31
Aceite/Accepted: 2014.09.20














