Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.38 no.2 Lisboa jun. 2015
ARTIGO
Potential of Mentha pulegium for mosquito control
Potencialidade da Menta pulegium no controlo de mosquitos
Diara Rocha1,3,*, Maria Novo1,3, Olívia Matos2,3, Ana C. Figueiredo4, Manuel Delgado5, Marilene D. Cabral6, Maria Liberato7 and Cristina Moiteiro8,*
1 Instituto de Higiene e Medicina Tropical, UNL, Rua da Junqueira, 100, 1349-008 Lisboa, Portugal. E-mails: dkrocha@fc.ul.pt, tenovo@ihmt.unl.pt
2 Instituto Nacional de Investigação Agrária e Veterinária, INIAV. Quinta do Marquês, Av. República, 2784-505 Oeiras, Portugal. E-mail: olivia.matos@iniav.pt
3 Unidade de Parasitologia e Microbiologia Médicas, IHMT, Rua da Junqueira, 100, 1349-008 Lisboa, Portugal.
4 Universidade de Lisboa, Faculdade de Ciências, Departamento de Biologia Vegetal-DBV, CESAM, Centro de Biotecnologia Vegetal, C2, Piso 1, Campo Grande, 1749-016 Lisboa, Portugal. E-mail: acfigueiredo@fc.ul.pt
5 Delegação do Ministério do Desenvolvimento Rural, MDR. Porto Novo, Santo Antão, Cabo Verde. E-mail: nayssdelgado.83@gmail.com
6 Universidade de Cabo Verde-UniCV, Campus Palmarejo, Praia, Cabo Verde. E-mail: marilena.cabral@docente.unicv.edu.cv.
7 Instituto de Investigação Científica Tropical, Jardim Botânico Tropical, Lg. dos Jerónimos, 1400- 209 Lisboa, Portugal Researcher emeritus. E-mail: m.c.liberato@sapo.pt
8 Universidade de Lisboa, Faculdade de Ciências, Departamento de Química e Bioquímica; Centro de Química e Bioquímica, Campo Grande, 1749-016 Lisboa, Portugal. E-mail: * cmoiteiro@fc.ul.pt, author for correspondence
ABSTRACT
Vector control remains an important strategy to fight mosquito-borne diseases, like malaria and dengue. Anopheles species are responsible for vast distribution of malaria, mainly in tropical areas, with dramatic infant morbidity and mortality. Aedes aegypti, the main vector of dengue, has a wide and expanding geographical distribution. It was found in Madeira Island, Portugal, in 2005, and in 2012 the first local dengue outbreak occurred. Also, in the African archipelago of Cape Verde, the first dengue epidemic in 2009, demonstrated that dengue virus is expanding. Phytochemicals offer not only effective mosquito control products, but are also biorational alternatives to organic synthetic pesticides. These chemicals from natural sources, with a high degree of biodegradation, are environmentally sound control agents. In the present study, Mentha pulegium essential oils (EOs) were assessed for larvicidal effects on third instar larvae of Anopheles atroparvus, Anopheles gambiae, Anopheles stephensi and Aedes aegypti (from Madeira and Cape Verde). The EOs chemical composition of M. pulegium from Portugal and Cape Verde was determined by 13C NRM and GC, GC-MS analysis. Larvicidal effect was observed on all species assayed with the strongest effect on Ae. aegypti from Cape Verde Islands.
Keywords: Aedes, Anopheles, vector control, essential oils.
RESUMO
O controlo vetorial continua preponderante no combate às doenças transmitidas por mosquitos, tais como malária e dengue. Anopheles spp. são responsáveis pela vasta distribuição de malária, sobretudo em áreas tropicais, causa de elevada morbilidade e mortalidade infantil. Aedes aegypti, o principal vetor da dengue, tem ampla distribuição geográfica, tendo sido encontrado na Ilha da Madeira, Portugal, em 2005 e em 2012 ocorreu o primeiro surto. Em Cabo Verde, a primeira epidemia de dengue ocorreu em 2009 demonstrando que o vírus da dengue continua em expansão. Fitoquímicos são potenciais alternativas aos pesticidas orgânicos sintéticos, sendo eficazes no controlo de mosquitos. Produtos químicos, obtidos a partir de fontes naturais, são agentes de controlo com elevado grau de biodegradabilidade, comportando menos riscos ambientais. Neste estudo, os óleos essenciais (OEs) de Mentha pulegium foram testados em larvas de terceiro estádio de Anopheles atroparvus, Anopheles gambiae, Anopheles stephensi e Aedes aegypti (da Madeira e Cabo Verde). A composição química dos OEs de M. pulegium de Portugal e Cabo Verde foi determinada por análise RMN de 13C, por CG e CG-EM. O efeito larvicida foi observado em todas as espécies, sendo maior nas larvas de Ae. aegypti de Cabo Verde.
Palavras Chave: Aedes, Anopheles, controlo vetorial, óleos essenciais.
Introduction
Mosquitoes are vectors of diseases like malaria, dengue and yellow fevers, Japanese encephalitis and filariasis (Gubler, 1998). More than 2.5 million people die from malaria each year, over 75% of them are African children, dengue fever is a debilitating and possibly fatal disease affecting between 50,000 to 100,000 people, being responsible for 25,000deaths/year (WHO, 2012). As a consequence of the public health burden, mosquito-borne diseases have a negative economic impact with loss in commercial and labor outputs, particularly in countries with tropical and subtropical climates. However, no part of the world is free from vector-borne diseases (Fradin and Day, 2002).
The strategies regarding control of mosquito vector populations assume highest importance in fighting these diseases. Actually, the use of synthetic insecticides for the management of vector populations is questioned, due to factors such as chemoresistance of vectors to insecticides and environmental hazards (Power, 2010). Pyrethroids are the main insecticides used in public health and have been used for decades for impregnation of mosquito bednets. However, pyrethroid resistance is already present in several major vectors of malaria in Africa (Sina and Aultman, 2001). The lack of an effective vaccine and the multiple resistances of parasites to anti-malaric drugs, evidence the importance of the malaria vectors control in the attempt to decrease malaria transmission.
In Western Europe, Anopheles atroparvus van Thiel was the most widespread malaria vector, particularly in the coastal lowlands. This species is a member of the Maculipennis Subgroup, which also includes mosquito vectors like Anopheles sacharovi Favre (Harbach, 2004). Anopheles atroparvus is generally considered zoophilic (Ponçon et al., 2007) and described as “very zoophilic” by Cambournac (1994), who also stated that its hosts, in order of preference, are rabbit, horse, cow, pig, sheep, and suggested that a long association between rabbit and An. atroparvus (since approx. 1000 BC) may be responsible for this hierarchy of preference.
Malaria transmission in Africa is mainly due to Anopheles gambiae s.l. and Anopheles funestus Giles. An. gambiae s.l. is considered to include the most efficient vectors of malaria in the world. It is a complex of seven species with Anopheles gambiae Giles s.s. and Anopheles arabiensis Patton, highly anthropophilic, being the major vectors (Besansky et al., 1994). Malaria vectors in Africa are characterized by differences in their biology, ecology and insecticides resistance, with consequent heterogeneous disease transmission and distinct epidemiological patterns.
Anopheles stephensi Liston is an important vector of human malaria throughout Middle East and South Asian regions, including the Indo-Pakistan sub-Continent, with a westward extension through Iran and Iraq into the Middle East and Arabian Peninsula. This species is considered to be the main malaria vector in the Persian Gulf area (Davidson, 1958). Behavioral studies of An. stephensi in the South of Iran have shown that it is highly zoophilic, although a wide range of anthropophilic indices (0.05–0.47) has been reported from the different geographical regions of Iran (Basseri et al., 2005) and India (Bruce-Chwatt et al., 1966).
According to the World Health Organization (WHO, 2012), Aedes aegypti Linnaeus is the primary vector of dengue. This mosquito is an invasive species, highly anthropophilic and adapted to human environments, promptly feeding on humans and breeding mainly in domestic or peridomestic recipients. A rapid rise in urban populations, without adequate urbanization, is placing a growing number of people in contact with the vector. Consequently, the number of dengue infections has increased worldwide over the last decades, with the occurrence of serious outbreaks with hemorrhagic fever. Dengue fever has become an important public health problem and about half of the world's population is now at risk (WHO, 2012).
Aedes aegypti populations' effective control, has proven to be extremely difficult. Lacking an effective vaccine, vector control methods, directed at both immature and adult mosquito populations, remain the primary method for reducing the risk of dengue transmission (Polsomboon et al., 2008). Control of adult mosquitoes using a variety of chemical means is fraught, with handicaps including high cost, slow operational response, low efficacy and development of insecticides resistance (Chuaycharoensuk et al., 2011).
Phytochemicals with proven mosquito control activity can be highly helpful in integrated mosquito vector control programs (Sukumar et al., 1991; Shaalan et al., 2005). Recently, essential oils and their components, in particular, are subject of increasing interest, as they are relatively safe for the environment as well as to human health, have a wide acceptance by consumers and potential for multi-purpose use (Ormancey et al., 2001). EOs have a broad spectrum of bioactivity due to the presence of several active constituents with different modes of action (Liu et al., 2006). Their lipophilic nature allows them to interfere with basic metabolic, biochemical, physiological and behavioral functions of insects.
Mentha L. is a genus of aromatic perennial herbs belonging to the family Lamiaceae, distributed mostly in temperate and sub-temperate regions of the world. Mentha pulegium L. (pennyroyal) is native in N Africa, W Asia, Caucasus to the Kazakhstan and Turkmenistan, and Europe (GRIN 2010). The pennyroyal is a creeping plant smaller than other Mentha spp. and spreads rapidly through its underground root system (Bradley, 1992). Stems are red-purple and highly branched, leaves are scale-like and flowers have verticillasters arrangement (Morales et al., 2010). Besides medicinal applications, insecticidal properties of several Mentha spp. EOs are reported against ants, mosquitoes, wasps, hornets and cockroaches (Conceição et al., 2010). Biological control of immature stages are thought to be the most powerful mean of reducing target population of dipteran and other agricultural pests (Rey et al., 1999).
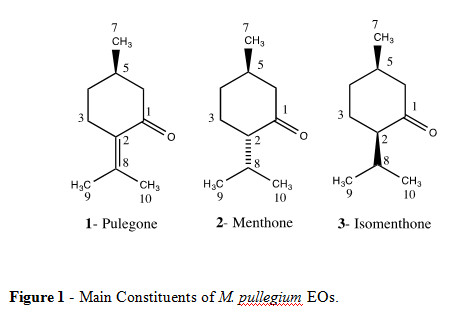
The monoterpene pulegone 1 (Figure 1) is a common constituent of Mentha spp. EOs, being referred as one main compound also in M. pulegium where it occurs at percentages ranging from 25 to 92% (Pino et al., 1996; Franzios et al., 1997; Lawrence, 1998; Aziz and Abbass, 2010).
This work intended to contribute to the evaluation of the potential use of pennyroyal EO in the control of malaria and dengue mosquito vector immatures, using plants from different geographical regions. Larvicidal effects of EOs of M. pulegium from Cape Verde and Portugal were assessed against mosquito vectors of malaria or dengue for the first time. Major compounds of these EOs were identified by 13C NMR spectroscopy, GC and GC-MS.
Material and methods
Plant material
Mentha pulegium aerial parts from Portugal were collected in Évora and near Braga, during springtime. At Cape Verde, collections were made at the same season in Santo Antão Island. Taxonomic identification of the plant samples was performed using morphological external characters according to Franco (1984) and Morales et al. (2010) and they were compared with verified herbarium specimens. Voucher samples of M. pulegium were deposited at the Herbarium of the “Instituto de Investigação Científica Tropical (IICT)”, Lisbon, with identification, collection site, collectors name and collection numbers: Mentha pulegium L., Portugal, Évora, Joaquim Russo, nº 1 and Mentha pulegium L., Cabo Verde, Santo Antão, Manuel Delgado, nº 1.
Mosquito colonies
Mosquito colonies of the several species tested had been reared continuously for some generations in laboratory, free of exposure to insecticides. They were maintained at 27±1 ºC and 70±5% relative humidity under a photoperiod of 12:12-h (light/dark) in the insectarium of the Medical Parasitology Unit of the “Instituto de Higiene e Medicina Tropical” (IHMT) in Lisbon. Larvae were fed with Tetra Menu® fish food. The adults were reared in humidified cages and supplied with 10% sucrose solution. Mosquito females were weekly given the opportunity to blood fed on anesthetized rats to allow eggs development and oviposition, as neither of these species is autogenic.
Colonies of Ae. aegypti from Cape Verde, (Santiago) and Madeira Archipelagos (Funchal) were raised from eggs locally obtained from this species females. These colonies (pupae and adult forms) were maintained in security cameras for manipulation.
Anopheles atroparvus colony was obtained from females collected in 1988 at Águas de Moura, Setúbal District (Portugal), after morphological observation of eggs to confirm species status. The colonies of An. gambiae strain Yaounde and An. stephensi strain SDA500 were both initiated with eggs from The Imperial College, and maintained at the insectarium of the “Centro de Malária e Outras Doenças Tropicais” (CMDT)/IHMT. Colonies of these two species were then installed in the Medical Parasitology Unit/IHMT from specimens gratefully provided by CMDT.
Essential oil isolation and chemical analysis
The EOs were isolated from M. pulegium aerial parts, by hydrodistillation for 3 h, using a modified Clevenger apparatus (EPCE 2010) and were stored in the dark at 4ºC until analysis. Pennyroyal EOs yield was calculated as the percentage of EO volume obtained by dry weight of plant used.
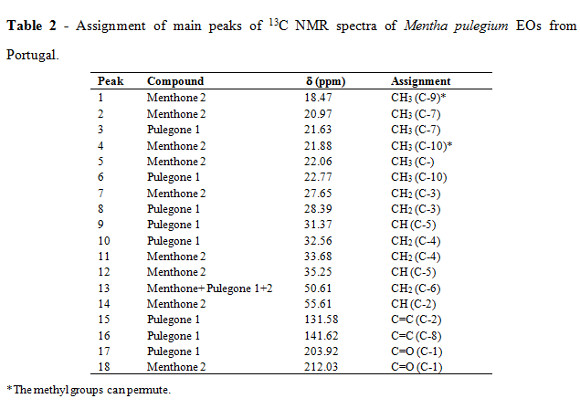
The most effective EOs samples were analyzed by 13C NMR to identify their major compounds. 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer operating at 100.61 MHz, equipped with a 5 mm QNP probe at room temperature. Each EO sample (100 mg) was dissolved in deuterochloroform (0.5 mL) and all chemical shift values given in ppm (d) refer to tetramethylsilane (TMS) as internal standard Chemical shifts and peaks attribution of 13C NMR spectra were made according to literature data (Kubeczka and Formáček, 2002), and pulegone and menthone standards.
Essential oils were analyzed by Gas Chromatography (GC), for component quantification, and Gas Chromatography coupled to Mass Spectrometry (GC-MS) for component identification, as detailed in Rocha (2014).
Assay procedures
Larvicidal activity of M. pulegium EOs against Anopheles spp. and Aedes aegypti was assessed by using WHO standard method (WHO, 1981). For bioassays, sets of 25 third late instar larvae were exposed to at least four concentrations, in three replicates, of each homogeneous oil-water suspensions according to standard WHO procedure, at 27±1ºC and 70±5% relative humidity. Negative controls were conducted for each series by adding 1 ml of water and Tween® 20 per 249 mL of water. Mortality data were recorded 24h and 48h after exposure. Larvae unable to reach water surface when touched were considered dead. Larval mortality was reported as the average of three replicates; mortality percentage rates were corrected using Abbott's formula (Abbott, 1925), and they were used to calculate LC50 and LC90 values within a confidence interval of 95% (WHO, 1981).
Preliminary studies allowed selecting the concentrations of EO obtained from M. pulegium collected near Braga: 5.0, 10.0, 25.0 and 45.0 mL L-1 for An. atroparvus, 10.0, 25.0, 50.0 and 80.0 mL L-1 for An. stephensi, and 30.0, 35.0, 40.0 and 50.0 mL L-1 for An. gambiae. Several concentration of EO from aerial parts of M. pulegium collected in Évora and Cape Verde (0.018, 0.020, 0.025, 0.030, 0.035 and 0.040 mL L-1) were evaluated against Aedes aegypti from Cape Verde and Madeira.
Statistical analysis
Data of EOs larvicidal effects were analyzed by computerized probit analysis, yielding a level of effectiveness of 50%, 90% and 99 % mortality at 95% confidence intervals (95% C.I.).
Results
Hydrodistillation of M. pulegium aerial parts produced intense yellow EOs with a yield of 0.3% (v/dry wt) from Cape Verde and 0.6 % (v/dry wt) from Portugal.
Preliminary results of M. pulegium EOs larvicidal effects against Ae. aegypti larvae from Cape Verde and Madeira Island (Table 1), showed that mosquitoes from Cape Verde, were more susceptible to pennyroyal EO (LC50 and LC99 of 0.107 mL L-1 and 0.209 mL L-1) than those from Madeira (LC50 and LC99 of 0.148 mL L‑1 and 0.319 mL L-1).
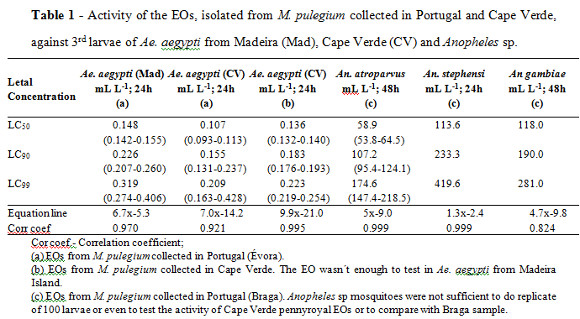
Bioassays using EOs isolated from pennyroyal collected in Braga showed low mortality rates on An. stephensi and An. gambiae larvae. Although it was tested a range of greater dilution in those species and it was not possible to test comparable amounts due to a lack of material (either mosquitoes or enough volumes of essential oils) the LC50 and 90 were higher for theses species. However, the results obtained against An. atroparvus larvae 48h after treatment allow considering the use of M. pulegium promissory for this species (Table 1).
The EOs of M. pulegium from Évora and Cape Verde exhibited high larvicidal activity against Ae. aegypti from Cape Verde, so chemical characterization by 13C NMR, GC and GC-MS were performed.
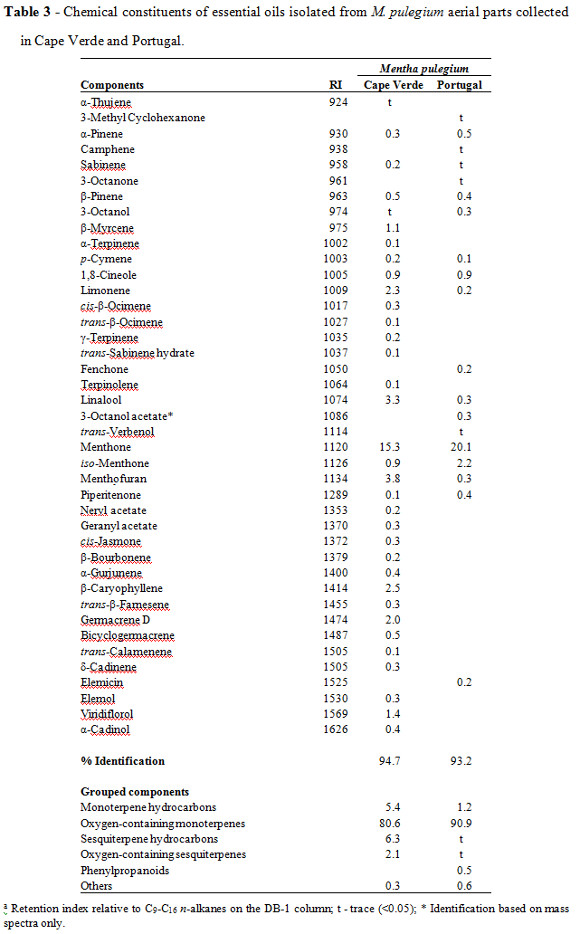
Qualitative 13C NMR analysis of this EO allowed the identification of the major compounds being pulegone 1 the main constituent, followed by menthone 2 and traces of its isomer, isomenthone 3 (Table 2, Figure 1). These results confirmed the characterization performed by GC and GC-MS, where pulegone (61%) and menthone (20%) were the main EO constituents (Table 3). The GC an GC-MS analyze of Cape Verde EO, revealed menthol (30%), menthone (15%), menthyl acetate (15%) as the main constituents, followed by pulegone (4%) (Table 3).
Discussion
Mentha pulegium EOs yield ranged between 0.3 and 0.6 % (v/dry wt.), which is in agreement with those reported by Kofidis et al. (2004), 0.1-2%, for the EO from wild M. spicata L., grown in Greece. Nevertheless, differences in the yield of Mentha EOs with respect to geographical regions were reported by Ijaz (2009).
Major differences were found between the evaluated M. pulegium EOs. Cape Verde M. pulegium EO main components were menthol (30%), menthone (15%) and menthyl acetate (15%), whereas Évora M. pulegium EO was mainly composed by pulegone (61%) and menthone (20%).
The data obtained from the analysis of M. pulegium EO from Portugal are in agreement with the results published by Lopes et al. (2010). In this study, the EOs from 18 M. pulegium samples from Portugal were evaluated, showing 96-97% of oxygenated monoterpenes, and the dominance of pulegone (66-87%), menthone (6-20%) and iso-menthone (0.4 to 17%). However, comparatively to M. pulegium EO from other populations also of Portugal, revealed different percentages of menthone and pulegone (Teixeira et al., 2012; Rodrigues et al., 2013). The constituents of M. pulegium EO have been subjected to a number of studies which have presented a variance in its constituents depending on the region of cultivation and there have been some differences in the components from different countries. It has been found that M. pulegium EO from Bulgaria contains essentially pulegone (43-45%); from Uruguay: pulegone (73%), isomenthone (13%); from Egypt: pulegone (44%), piperitone (12%); from Tunisia: pulegone (42%), isomenthone (11%) Boukhebti et al. (2011).
Mentha pulegium EOs from Portugal showed high larvicidal effects against Ae. aegypti from Cape Verde (LC50 and LC99 of 0.107 mL L-1 and 0.209 mL L-1) and Anopheles stephensi (Table 1) with registered mortality 24-h after contact and 100% mortality 48-h after treatment (result not presented in Table 1).
Ae. aegypti from Cape Verde seemed to be more susceptible to pennyroyal EOs than the same species introduced in Madeira from Venezuela (Seixas et al., 2013) which could be related to intrinsic genetics character of mosquito. The bioactivity of phytochemicals against mosquito larvae can vary significantly depending on plant species, plant part and age of plant part, solvent used in extraction and mosquito species (Sukumar et al., 1991).
Pennyroyal EOs is reported as bioactive against fleas, ants, lice, mosquitoes, ticks and moths. Pulegone from M. pulegium is considered one of the most active constituents against insects. Nevertheless reports on menthone studies against insects are well known (Koul et al., 2008). In this study menthone exhibited very low activity against 3rd instar larvae and pulegone high activity (results not shown). Although pulegone and menthone are reported as bioactive and useful to pest control (Verdian-Rivi, 2008) scarce references to their larvicidal effects on mosquito vectors of malaria and dengue were find.
Although the World Health Organization (WHO) does not specify any criteria for classifying the larvicidal potential of new products, some authors use the values of the minimum lethal concentration that eliminates 50% of the population (LC50) as a criterion for activity. Specifically, if LC50<50 mg L-1 the product is considered very active, if 50 < LC50 < 100 mg L‑1 the product is considered active, and when LC50 > 750 mg L-1 the product is considered inactive (Komalamisra et al., 2005; Magalhães et al., 2010; Dias et al., 2014). Based on this norm the pennyroyal EOs from Cape Verde and Portugal are in the class of active. However, the highest larvicidal activity corresponds to the EO from Portugal pennyroyal (LC50= 0.107 mL L-1, which is equivalent to 20.1 mg L-1).
Mortality of larvae exposed to “insecticidal” compounds may be the result of several details, including structure, function, and biochemistry of the insect cuticle in relation to the molting cycle. It is well known that many EOs and their constituents affect biochemical processes, which specifically disrupt the endocrinologic balance of insects. They may be neurotoxic or may act as insect growth regulators, disrupting the normal process of morphogenesis (Balandrin and Klocke, 1988). According to Cantrell et al. (2010), larvicide compounds act by absorption through the cuticle, via the respiratory tract, and/or enter by ingestion via the gastrointestinal tract. Once in the interior of the larva, the substances can reach the site of action or can cause systemic effects by diffusion into different tissues (Souza et al., 2012).
Conclusion
Present results are encouraging and clearly demonstrate the potential of M. pulegium essential oil as possible larvicidal against Aedes aegypti and Anopheles spp.. Further studies have to be focus on bioassay with isolates standing in phytochemical laboratories in that the mode of action of bio products will be evaluated and there are certainly many interesting activities yet to be discovered.
Acknowledgements
This work was supported by Diara Rocha PhD Grant, Fundação Calouste Gulbenkian (Neglected Tropical Diseases for PALOP students) by UPMM/IHMT funds and National Funds through the FCT under Pest-OE/EQB/LA0023/2011 and Pest-OE/QUI/UI0612/2013.
References
Abbott, W.S. (1925) - A method of computing the effectiveness of an insecticide. Journal of Economy Entomology, vol.18, n. 1, p. 265-267. [ Links ]
Aziz, E.E. and Abbass, M.H. (2010) - Chemical composition and efficiency of five essential oil against Callosobruchus maculates (F.) on Vigina radiate seeds. American Eurasian Journal Agriculture Environmental Science, vol. 8, n. 4, p. 411-419. [ Links ]
Balandrin, M.F. and Klocke, J.A. (1988) - Medicinal, aromatic, and industrial materials from plants, In: Bajaj, Y.P.S. (Ed.). Biotechnology in agriculture and forestry, medicinal and aromatic plants I. Berlin, Heidelberg, Springer-Verlag, p. 3-36. [ Links ]
Basseri, H.R.; Moosakazemi, S.H.; Yousefi, S.; Mohebali, M.; Hajaran H. and Jedari, M. (2005) - Anthropophly of malaria vectors in Kahnooj district, south of Kerman, Iran. Iranian Journal Public Health, vol. 34, n. 2, p. 27-35. [ Links ]
Besansky, N.J.; Powell, J.R.; Caccone, A.; Hamm, D.M.; Scott, J.A. and Collins, F.H. (1994) - Molecular phylogeny of the Anopheles gambiae complex suggests genetic introgression between principal malaria vectors. Proceedings of the National Academy of sciences of the United States of America, vol. 91, n.15, p. 6885-6888. [ Links ]
Boukhebti, H.; Chaker, A.N.; Belhadj H.; Sahli, F.; Ramdhani, M.; Laouer, H. and Harzallah, D. (2011) - Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Der Pharmacia Lettre, vol. 3, n. 4, p. 267-275. [ Links ]
Bradley, P.R. (1992) - British herbal compendium, vol. 1. Bournemouth, British Herbal Medicine Association, 239 p. [ Links ]
Bruce-Chwatt, J.L.; Garett-Jones, C. and Weitz, B. (1966) - Ten years study (1955-1964) of host selection by Anopheline mosquitoes. Bulletin World Health Organization, vol. 35, n. 2, p. 405-439. [ Links ]
Cambournac, F.J. (1994) - Contribution to the history of malaria epidemiology and control in Portugal and some other places. Parassitologia, vol. 36, n 1-2, p. 215-222. [ Links ]
Cantrell, C.L.; Pridgeon, J.W.; Fronczek, F.R. and Becnel, J.J. (2010) - Structure activity relationship studies on derivatives of Eudesmanolides from Inula helenium as toxicants against Aedes aegypti larvae and adults. Chemistry Biodiversity, vol. 7, n. 7, p. 1681–1697. [ Links ]
Chuaycharoensuk, T.; Juntarajumnong, W.; Boonyuan, W.; Bangs M.J.; Akratanakul, P.; Thammapalo S.; Jirakanjanakit, N.; Tanasinchayakul, S. and Chareonviriyaphap T. (2011) - Frequency of pyrethroid resistance in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Thailand. Journal of Vector Ecology, vol. 36, n. 1, p. 204-212. [ Links ]
Conceição, C.; Matos, O.; Barbosa, A. and Mexia, A. (2010) - Potential of plant products as protectants of stored maize against Sitophilus zeamais Motschulsky (Coleoptera:Curculionidae). In: Proceedings 20th IWCSPP, 425, p. 618-624. [ Links ]
Davidson, G. (1958) - Studies on insecticide resistance in anopheline mosquitoes. Bulletin World Health Organization, vol. 18, n. 4, p. 579-621. [ Links ]
Dias, C.N. and Moraes, D.F.C. (2014) - Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: review. Parasitology Resource 113, vol 2, p. 565-92. [ Links ]
EPCE (2010) - European Pharmacopoeia by Council of Europe, European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & Healthcare, 7th Edition. Strasbourg. [Cited 1st July of 2013]. Available from http://www.worldcat.org/search?q=au%3AEuropean+Directorate+for+the+Quality+of+Medicines+%26+Healthcare.&qt=hot_author2010. [ Links ]
Fradin, M.S. and Day, J.F. (2002) - Comparative efficacy of insect repellents against mosquitoes bites. The New England Journal of Medicine, vol. 347, n. 1, p. 13-18. [ Links ]
Franco, J.A. (1984) - Nova Flora de Portugal (Continente e Açores), vol. 2. Lisboa, Soc. Astória Lda, 660 p. [ Links ]
Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. (1997)- Insecticidal and genotoxic activities of pennyroyal essential oils. Journal Agriculture Food Chemistry, vol. 45, n. 7, p. 2690-2694. [ Links ]
GRIN. Germplasm Resources Information Network (Online Database) (2010) - National germplasm resources laborator (on line). Beltsville, Maryland. USDA, ARS, National Genetic Resources Program. [Cited 5th July of 2013]. Available at: http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl24079. [ Links ]
Gubler, D.J. (1998) - Resurgent vector borne diseases as a global health problem. Emerging Infecious Diseases Journal, vol. 4, n. 3, p. 442-450. [ Links ]
Harbach, R.E. (2004) - The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bulletin of Entomological Research, vol. 94, n. 6, p. 537-553. [ Links ]
Ijaz, H.A. (2009) - Characterization and biological activities of essential oils of some species of Lamiaceae. (Phd Thesis). Faisalabad, Pakistan, University of Agriculture, 365 p. [ Links ]
Kofidis, G.; Bosabalidis, A. and Kokkini S. (2004) - Seasonal variation of essential oils in a linalool rich chemotype of Mentha spicata grown wild in Greece. Journal of Essential Oil Research, vol. 16, n. 5, p. 469-472. [ Links ]
Komalamisra, N., Trongtokit, Y., Rongsriyam, Y. and Apiwathnasorn, C. (2005) - Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian. Journal of Tropical Medicine Public Health Vol. 36, p. 1412-1422. [ Links ]
Koul, O.; Walia, S. and Dhaliwal, G.S. (2008) - Essential oils as green pesticides: Potential and constraints. Biopesticide International, vol. 4, n. 1, p. 63–84. [ Links ]
Kubeczka, K.-H. and Formáček, V. (2002) - Essential Oils Analysis by Capillary Gas Chromatography and Carbon-13 NMR Spectroscopy, 2nd Ed., New York, John Wiley & Sons, Ltd., 480 p. [ Links ]
Lawrence, B.M. (1998) - Progress in essential oils. Perfumer and flavorist, vol. 23, p. 63-68. [ Links ]
Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H. and Shen, Y.F. (2006) - Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresource Technology, vol. 97, n. 15, p. 1969-1973. [ Links ]
Lopes V.R; Barata A.M.; Rocha F., Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. (2010) – Morphological and Chemical variability assessment for Portuguese Mentha pulegium L (pennyroyal) accession. In: Proceedings of the VIII International Ethnobotany Symposium, Lisboa, Portugal, p. 573-583. [ Links ]
Magalhães, L.A.M.; Lima, M.P.; Marques, M.O.M.; Facanali, R., Pinto, A.C.S. and Tadei, W.P. (2010) - Chemical composition and larvicidal activity against Aedes aegypti larvae of essential oils from four Guarea species. Molecules. Vol. 15, n.8, p 5734-5741. [ Links ]
Morales, A.; Quintanar, A. and Cabezas, F. (2010) - Labiatae. In: Castroviejo, S. (ed.) - Flora Ibérica. Plantas vasculares de la Península Ibérica e Islas Baleares., vol. 12. Madrid, Real Jardín Botánico, C.S.I.C., p. 25-484. [Cited 10th June of 2013]. Available from: http://www.floraiberica.es/floraiberica/texto/pdfs/12_140_00_LABIATAE . [ Links ]
Ormancey, X.; Sisalli, S. and Coutiere, P. (2001) - Formulation of essential oils in functional perfumery. Parfums Cosmetiques Actualites, vol. 157, p. 30-40. [ Links ]
Pino, J.A.; Rosado, A. and Fuentes, V. (1996) - Chemical composition of the essential oil of Mentha pulegium L. from Cuba. Journal Essential Oil Resource, vol. 8, n. 3, p. 295-296. [ Links ]
Polsomboon, S.; Grieco, J.P.; Achee, N.L.; Chauhan, A.R.; Tanasinchayakul, S.; Pothikasikorn, J. and Chareonviriyaphap T. (2008) - Behavioral responses of catnip (Nepeta cataria) by two species of mosquitoes, Aedes aegypti and Anopheles harrisoni, in Thailand. Journal American Mosquito Control Association, vol. 24, n. 4, p. 513-519. [ Links ]
Ponçon, N.; Toty, C.; L'Ambert, G.; Le Goff, G.; Brengues, C.; Schaffner, F. and Fontenille, D. (2007) - Biology and dynamics of potential malaria vectors in Southern France. Malaria Journal, vol. 6, 18 p. [ Links ]
Power, A.G. (2010) - Ecosystem services and agriculture: trade-offs and synergies. Philosophical Transaction Royal Society, vol. 365, n. 1554, p. 2959-2971. [ Links ]
Rey, D.; Pautou, M.P. and Meyran, J.C. (1999) - Hystopathological effects of tannic acid on the midgut epithelium of some aquatic Diptera larvae. Journal Invertebrate Pathology., vol. 73, n. 2, p.173-181. [ Links ]
Rocha, D.K., (2014) - Plantas medicinais tropicais e mediterrânicas com propriedades biocidas no controlo de insetos vetores de agentes patogénicos. Tese de Doutoramento, Universidade Nova de Lisboa, IHMT, Lisboa 181p. [ Links ]
Rodrigues, L.; Póvoa, O.; Teixeira, G.; Figueiredo, A.C.; Moldão, M. Monteiro, A. (2013) - Trichomes micromorphology and essential oil variation at different developmental stages of cultivated and wild growing Mentha pulegium L. populations from Portugal. Industrial Crops and Products, vol. 43, p. 692– 700 [ Links ]
Shaalan, E.; Canyon, D.V., Younes, M., Abdelwahab, H. and Mansour, A. (2005). A review of botanicals with mosquitocidal potential. Environment International vol. 31, n. 8, p. 1149-1166. [ Links ]
Seixas, G., Salgueiro, P., Silva, A:C., Campos, M., Spenassatto, C., Reyes-Lugo, M., Novo, M.T., Ribolla, P.E.M., Pinto, J.P.S.S. and Sousa, C.A. (2013). Aedes aegypti on Madeira Island (Portugal): genetic variation of a recently introduced dengue vector. Memórias do Instituto Oswaldo Cruz Dec; 108 (Suppl 1): 3–10 [ Links ]
Sina, B.J. and Aultman, S.K. (2001) - Resisting resistance. Trends Parasitology, vol. 17, n. 7, p. 305-306. [ Links ]
Souza, T.M.; Cunha, A.P.; Farias D.F.; Machado L.K.; Morais S.M.; Ricardo N.M.P.S. and Carvalho A.F.U. (2012) - Insecticidal activity against Aedes aegypti of m-pentadecadienyl-phenol isolated from Myracrodruon urundeuva seeds. Pest Managements Science, vol. 68, n. 10, p. 1380-1384. [ Links ]
Sukumar, K.; Perich, M.J. and Boobar, L.R. (1991) - Botanical derivatives in mosquito control: a review. Journal American Mosquito Control Association., vol. 7, n. 2, p. 210-37. [ Links ]
Teixeira, B.; Marques, A.; Ramos, C.; Batista, I. Serrano, C.; Matos, O.; Nerg, N.; Nogueira, J.M.F.; Saraiva, J.A. and Nunes, M.L. (2012). European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and anti-microbial properties of extracts and essential oil. Industrial Crops and Products, Vol. 36, p. 81-87. [ Links ]
Verdian-Rivi M. (2008) - Effect of the essential oil composition and biological activity of Ziziphora clinopodiodes Lam. on the against Anopheles Stephensi and Culex pipiens Parva from Iran. Saudi Journal of Biological Sciences, vol. 15, n.1, p. 185-188. [ Links ]
WHO-World Health Organization (1981) - Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. Geneva, WHO, 6 p. [ Links ]
WHO-World Health Organization (2012) - World Malaria Report 2012. Geneva, WHO, 288 p. [ Links ]
Received/Recebido: 2014.11.19
Accepted/Aceite: 2015.05.02














