Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.41 no.4 Lisboa dez. 2018
https://doi.org/10.19084/RCA16135
ARTIGO
Potassium nitrate priming mitigates salt stress on wheat seedlings
Nitrato de potássio ameniza o estresse salino em plântulas de trigo
Fábio Steiner1*, Alan M. Zuffo1, Carlos E. da Silva Oliveira1, Guilherme B. Honda2 and Juan S. Machado2
1 Universidade Estadual de Mato Grosso do Sul, Dpto. de Produção Vegetal, Unidade Universitário de Cassilândia, MS 306, km 6,4, CEP: 79540-000, Cassilândia, MS, Brasil
2 Faculdades Integradas de Ourinhos, Dpto. de Agronomia, Rodovia BR 153, km 338,42 – CEP 19.909-100, Ourinhos, SP, Brasil
(* E-mail: steiner@uems.br)
ABSTRACT
Seeds of wheat (Triticum aestivum L., cv. Jadeíde 11) were used to investigate the effects of potassium nitrate priming on germination and early seedling growth under salt stress. It was hypothesized that priming with potassium nitrate may improve seed germination and plant establishment by mitigating the negative effects of saline stress through its role in cell osmotic balance. The seeds were soaked in distilled water or in a 10 g L–1 KNO3 solution at 25 °C for 2 hours, and after drying, were distributed in plastic boxes with blotter paper containing different salt solutions prepared with concentrations of 0 (control), 25, 50, 75 and 100 mmol L–1 NaCl. The plastic boxes were kept in a seed germinator, at 22 °C for 12 days. A completely randomized design in a 2 × 5 factorial scheme with four replications of 50 seeds each was used. The primed seeds with KNO3 showed improved germination performance, early growth and vigor index of wheat seedlings in salt stress conditions. Low salt concentrations may induce osmotic adjustment activity in the wheat plants and lead to increases in shoot and root length of wheat seedlings, whereas higher concentrations cause severe inhibition of plant growth. The ‶Jadeíde 11″ wheat cultivar is a moderately tolerant genotype to salt stress during the seedling establishment stage by presenting yield stability index greater than 0.74 until the level of 100 mmol L–1 of NaCl, and therefore can be recommended for cropping in soils with high salinity levels.
Keywords: Triticum aestivum, salinity, osmotic potential, NaCl, KNO3, seed treatment.
RESUMO
Sementes de trigo (Triticum aestivum L., cv. Jadeíde 11) foram utilizados para investigar os efeitos do condicionamento osmótico com nitrato de potássio na germinação e no crescimento inicial das plântulas sob estresse salino. Supõe-se que o condicionamento com nitrato de potássio pode melhorar a germinação das sementes e o estabelecimento das plantas por amenizar os efeitos negativos do estresse salino através do seu papel no equilíbrio osmótico das células. As sementes foram embebidas em água destilada ou em solução contendo 10 g L–1 de KNO3 à 25 °C durante 2 horas e, após secagem, foram distribuídas em caixas plásticas com papel mata-borrão contendo diferentes soluções salinas preparadas com concentrações de 0 (controle), 25, 50, 75 e 100 mmol L–1 de NaCl. As caixas plásticas com as sementes foram mantidas em germinador, à 22 °C durante 12 dias. Foi utilizado o delineamento experimental inteiramente casualizado, em esquema fatorial 2 x 5, com quatro repetições de 50 sementes. Sementes de trigo condicionadas com nitrato de potássio possuem maior taxa de germinação, crescimento inicial e índice de vigor de plântulas quando submetidas em condições de estresse salino. Níveis baixos de salinidade podem induzir o ajustamento osmótico nas plântulas de trigo e resultar em maior comprimento da parte aérea e das raízes, enquanto que altos níveis de salinidade causam severa inibição do crescimento das plantas. O cultivar de trigo "Jadeíde 11" é um genótipo moderadamente tolerante ao estresse salino durante a fase de estabelecimento das plântulas, apresentando índice de estabilidade de rendimento superior a 0,74 até o nível de 100 mmol L-1 de NaCl e, portanto, pode ser recomendado para o cultivo em áreas com altos níveis de salinidade do solo.
Palavras-chave: Triticum aestivum, salinidade, potencial osmótico, NaCl, KNO3, tratamento de semente.
INTRODUCTION
The sustainability of agriculture production in many areas of the world including North and South America, Asia, Europe and Australia is at risk due to soil salinization (Chaves et al., 2006). Salinity caused by excessive salts in the soil solution or in irrigation water is one of the major environmental stresses that limit plant growth and crop yield in arid, semiarid and other areas of the world. One-third of the worlds cultivated land, 7% of the total world land and 50% of the irrigated land is affected by salinity (Zhu, 2001). Salt-affected soil may be grouped into four categories based on the electrical conductivity of the extract (ECe) from the water saturated soil, i.e., slightly saline (ECe 2 to 4 dS m–1), moderately saline (ECe 4 to 8 dS m–1), highly saline (ECe 8 to 16 dS m–1) and extremely saline (ECe > 16 dS m–1) (Richards et al., 1954). Conventionally, however, saline soils are defined as those having an ECe value ≥ 4 dS m–1 or 40 mmol L–1 NaCl (Richards et al., 1954).
Excessive salt concentrations in the soil solution may adversely affect plant growth either through osmotic inhibition of water uptake by plant roots or specific ion effects (Parida and Das, 2005). Salinity reduces cell turgor and depresses rates of root and leaf elongation (Memon et al., 2010), suggesting that environmental impact of excess salt acts primarily on water uptake. Specific ion effects may cause mineral nutrition disorders due to competitive absorption of ions or direct toxicity (Feijão et al., 2011). Nutritional imbalance caused by salinity is mainly due to the reduction in the uptake and assimilation of essential elements to the plants (Parida and Das, 2005; Munns and Tester, 2008; Feijão et al., 2011).
Salinity affects plant growth at all developmental stages; however, sensitivity varies from one growth stage to another. Seed germination is one of the most fundamental and vital phases in the growth cycle of plants that determine plant establishment and grain yield of crops. Delayed and reduced seedling emergence cause non-uniform stand establishment, which result in yield reductions (Lawles et al., 2012). Therefore, a greater salt stress tolerance could improve the plant establishment and grain yield stability of wheat in saline soils.
Among the strategies used to mitigate the salt stress-induced adverse effects the pretreatment of seeds with salts or plant growth regulators are cited as the most appropriate, efficient and economic techniques to improve seed germination in saline soils (Mohammadi, 2009; Kaya et al., 2013; Oliveira and Steiner, 2017). Indeed, seed-priming treatments using salts such as potassium nitrate (KNO3) have been shown to have beneficial effects on germination, growth and yield of a wide range of plant species under improper conditions (Anosheh et al., 2010; Ahmadvand et al., 2012; Fuller et al., 2012; Zanotti et al., 2013). Kaya et al. (2006) indicated that priming of sunflower seeds with KNO3 led to increasing of germination percentage in drought and salinity stresses. Mohammadi (2009) found that among the priming treatments, primed soybean seeds with KNO3 showed the highest values for all traits when compared to unprimed seeds. This author reported increase on the germination percentage, germination rate and seedling dry matter from 28.3%, 129.4% and 58.1%, respectively.
The exogenous application of KNO3 can stimulate seed germination at abiotic stress conditions due to the production of substances that release nitric oxide (NO) (Kaiser et al., 2016; Parankusam et al., 2017). These substances act in membrane permeability, preventing or reversing the damage caused by environmental stresses (Pereira et al., 2010). Therefore, as the process of saline and water stress involves changes in osmotic potential and tension on the cell membrane, it is possible that substances NO-liberating improves the germination process under salt stress (Kaiser et al., 2016). Nitric oxide is a molecule that acts as a signaler in higher plants and studies on their functions in the physiological processes of plants indicate that NO is involved in the regulation of plant growth and development, defense against pathogen and responses to abiotic stress (Sanz et al., 2015). However, the effectiveness of pretreatment of wheat seeds with KNO3 in improving germination rate and initial growth of the plants under salinity conditions are still incipient and inconclusive (Fuller et al., 2012).
This research was carried out to investigate the possibility of reducing the negative effects of salt stress on seed germination and early growth of wheat (Triticum aestivum L.) by seed priming with potassium nitrate.
MATERIAL AND METHODS
Plant material and treatments
Seeds of wheat (Triticum aestivum L., cv. Jadeíde 11) were sterilized with sodium hypochlorite solution (1%, v/v) for 5 minutes and washed immediately with distilled water many times. The sterilized seeds were then subjected to priming by direct immersion in distilled water (control) or in a 10 g L–1 KNO3 solution for 2 hours at 25 °C. After priming period, seeds were put to dry in plastic boxes (11.0 × 11.0 × 3.5 cm, type Gerbox) with blotter paper at room temperature (24–28 °C) for 48 hours, and then subjected to five levels of salinity [0 (control), 25, 50, 75 and 100 mmol L–1 of NaCl]. Treatments were arranged in a completely randomized design in a 2 × 5 factorial: two priming techniques (0 or 10 g L–1 of KNO3) and five salinity levels, with four replications.
Germination and growth conditions
Four replicates of 50 seeds were evenly distributed in plastic boxes with blotter paper, properly moistened with the salt solution of each treatment, in a volume equivalent to 2.5 times the weight of dry paper. The boxes were then closed with lids to prevent evaporation and maintain the relative humidity close to 100%. Germination was carried out in a germination chamber under 12/12 h photoperiod (light/darkness), light fluence of 80 μmol m−2 s−1 photosynthetic photon flux density (PPFD) and temperature of 22 °C for 12 days. Seeds were considered germinated when radicle were longer than 5.0 mm. Germinated seeds were recorded every 24 h for 12 days.
Measurements of germination and seedling growth
The germination (G), germination rate index (GRI), mean germination time (MGT) of wheat seeds were measured. The equations 1–3 and the parameters therein were employed to calculate the parameters of seed germination.
G (%) = SNG / SN0 × 100 [Eq. 1]
where G is germination, SNG is the number of germinated seeds, and SN0 is the number of experimental seeds with viability (50 seeds).
GRI = Σ (ni / ti) [Eq. 2]
where GRI is the germination rate index (seed day–1), ni is the number of germinated seeds on a given day, and ti is the time in days from the starting/sowing day (0) (Maguire, 1962).
MGT = (Σniti) / Σni [Eq. 3]
where MGT is the mean germination time (day), ni is the number of germinated seeds on a given day, and ti is the time in days from the starting/sowing day (0) (Labouriau, 1983).
The shoot and radicle length was measured in 20 normal seedlings randomly obtained after count of the total germination (12th day) using meter scale. The results were expressed in centimeter (cm). For the determination of dry matter production of shoot and roots, all seedlings obtained at the end of the germination test (12 days) were separated into shoots and roots, dried in a forced air circulation oven for three days at 65 ºC, and then weighed. The results were expressed in mg seedling–1. To determine the root: shoot ratio (RSR), root dry matter obtained was divided by the shoot dry matter.
After measuring of seedling, length and dry matter traits, the seedling vigor index and salt tolerance index, were calculated using the by following equations:
SVI = SL × Σ (ni / ti) [Eq. 4]
where SVI is seedling vigor index, SL is the shoot length in the twelfth day (cm), ni is the number of germinated seeds on a given day, and ti is the time in days from the starting/sowing day (0) (Zhang et al., 2007).
YSI = YS / YC [Eq. 5]
where YSI is the yield stability index, YS and YC are the total dry matter yield (mg per seedling) under saline stress and non-stress conditions (NaCl-free treatment), respectively (Bouslama and Schapaugh, 1984).
Statistical analyses
The normality of data was previously tested by the Kolmogorov-Smirnov test and then submitted to analysis of variance (ANOVA), and means of priming treatments were compared by Fisher's Least Significant Difference (LSD) test at the 0.05 level of confidence. For the salinity levels were used polynomial regression analysis and significant equations with the higher coefficient of determination (F test; P ≤ 0.05) were adjusted. For statistical analysis, the data expressed in percentage were previously transformed into arc sin. All analyses were performed using Sisvar version 5.6 software for Windows (Statistical Analysis Software, UFLA, Lavras, MG, BRA) (Ferreira, 2011).
RESULTS AND DISCUSSION
A summary of the analysis of variance for the measurements of germination, vigor index and seedling growth inhibition of wheat is shown in Table 1. The results of the analysis of variance showed significant effects (P<0.05) for the main effects of seed priming with potassium nitrate and salinity levels, as well as for interaction, for many of the traits measured (Table 1). The significant interaction between the main effects of potassium nitrate priming and salt stress for most of the evaluated characteristics indicates germination and growth of wheat seedlings from seeds subjected to priming of potassium nitrate have different response with regard to the salinity level compared primed seeds with water (control).
Effect of potassium nitrate priming and salt stress on seed germination
The germination percentage of wheat seeds (Figure 1A) was higher than the standard values (i.e., 80%) for the commercialization of wheat seeds in Brazil (MAPA, 2013), indicating that the seeds used in this study were of high physiological quality.
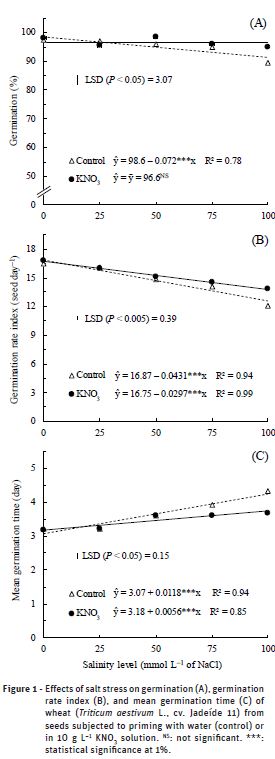
The germination response of seeds submitted to water priming (control) was significantly affected by salt stress induced by NaCl solutions (Figure 1A). The inhibiting action of saline stress on wheat germination was increased with the rise of salinity levels, and the exposure of seeds to 100 mmol L–1 NaCl reduced the mean germination by 7.30 percentage points (from 98.6% to 91.4%) compared to the NaCl-free treatment. However, when the seeds were submitted to KNO3 priming, the germination percentage was not significantly affected by saline stress induced by NaCl solutions (Figure 1A). Additionally, when the seeds were exposed to high levels of salinity, KNO3 priming also resulted in higher germination rate index (Figure 1B) and lower mean germination time (Figure 1C) compared to water priming. These results show that priming of wheat seeds with KNO3 can have significant improvements in the process of germination under saline conditions. Similarly, Fuller et al. (2012) concluded that the use of priming techniques can enhance the germination of wheat seed under saline conditions and under conditions of mild salt stress priming can entirely overcome the effect salt. These findings agree with Kubala et al. (2015) who found that seeds primed with an osmotic solution may improve germination performance through metabolic activation involving the synthesis of proteins, nucleic acids, and enzymes, and increasing water uptake, respiratory activity and reserve mobilization. Therefore, in field conditions where the soil is affected by high salinity levels, the use of seeds primed with KNO3 could make the difference between successful field germination and establishment or substantial crop failure.
Under salt stress conditions the KNO3 priming can improve seed germination due to the production of substances that release nitric oxide (NO) (Kaiser et al., 2016). According to Pereira et al. (2010), these substances NO-liberating act in membrane permeability, preventing or reversing the damage caused by osmotic stresses. Nitric oxide is a molecule that acts as a signaler in higher plants and it is involved in the regulation of plant growth and development, defense against pathogen and responses to abiotic stress (Sanz et al., 2015).
The high germination percentage values of wheat seeds (>91%) under conditions of high salt levels indicate that this crop is a moderately tolerant species to saline stress during the phase of seed germination, confirming the results reported by Maas and Hoffman (1977). In oat seeds, Brunes et al. (2013) found that salinity levels above 50 mmol L–1 NaCl completely inhibited the germination of seeds, indicating that the two oat cultivars used are susceptible to salt stress. Germination represents a fundamental stage of plant's life highly responsive to environmental conditions. Therefore, greater tolerance of crops to high salt levels could improve the stability of crop yield under saline conditions. According to Brunes et al. (2013), the salt stress can completely inhibit seed germination at higher levels or induces a state of dormancy at low levels, as well as reduce imbibition of water because of lowered osmotic potentials of the medium and causes changes in metabolic activity. Salt stress affects seed germination through osmotic effects, ion toxicity or a combination of the two effects (Parida and Das, 2005; Munns and Tester, 2008; Feijão et al., 2011).
The germination rate index (GRI) was reduced linearly with increasing salt levels up to 100 mmol L–1 NaCl in both priming treatments (water or KNO3), however, this reduction was more pronounced in seeds subjected to water priming (Figure 1B). At 100 mmol L–1 NaCl the germination rate index was 12.56 seed day–1 in seeds primed with water (control), and was significantly greater (13.78 seed day–1) when seeds were primed with KNO3. The decrease in GRI was due to lower capacity of water uptake by the seeds with highly negative osmotic potential. The lower germination rate and growth inhibition due to salinity are caused by low external water potential, ion imbalance and specific ion toxicity (Munns and Tester, 2008; Feijão et al., 2011). Under these conditions, there is a decrease in water uptake and an excessive uptake of ions (Akram et al., 2010). Osmotic stress affects the starch synthesis reactions and energy production process (adenosine triphosphate – ATP) through respiration, resulting in reduced of germination percentage (Figure 1A), germination rate index (Figure 1B) and thus in delay of germination (Figure 1C).
The mean germination time (MGT) was delayed with the rise of salinity levels (Figure 1C). At 100 mmol L–1 the MGT was delayed 1.18 days in the primed seeds with water (control), against 0.56 days in the KNO3 priming compared to the NaCl-free treatment. A delay in the mean time to germination may be disadvantageous for successful establishment, since the delayed germination leaving the seeds more vulnerable to attack from predators (pests and pathogens) and, therefore, compromise the establishment of a uniform stand.
The delay of germination was due to salinity affect the water uptake of the seeds, which is the first step to occur germination process (i.e., imbibition). According to Marcos-Filho (2005), it is necessary that the seeds reach an adequate level of hydration during the imbibition phase, to occur reactivation of seed metabolic processes and growth of embryonic axis. Seeds subjected to osmotic stress require more time to adjust the internal osmotic potential in accordance with the external environment (Parida and Das, 2005; Munns and Tester, 2008). Meneses et al. (2011) reported that highly negative osmotic potential may affect the seeds water uptake, making germination not possible. Additionally, the osmotic potential of the external medium can affect the enzymatic reactions in the seed, therefore, the delay in germination is due to delay of enzymatic reactions (Marcos-Filho, 2005), caused by the break of the imbibition period. This inference agrees with other observations in wheat, where salinity has been shown to negatively affect the rate of starch remobilization by causing a decrease in α-amylase activity (Almansouri et al., 2001). The most common responses of plants to reduction of osmotic potential are a delay in initial germination and a reduction in the rate and total germination (Oliveira and Gomes-Filho 2009; Gordin et al., 2015). The result of these changes is an unevenness in the germination process and stand establishment.
Effect of potassium nitrate priming and salt stress on seedling growth
The early growth of wheat was greater in low salinity conditions; however, with increasing salt stress level the growth of shoots and roots of wheat were severely inhibited (Figure 2). When the seeds were primed with KNO3, the shoot length increased from 14.7 cm in the NaCl-free treatment to maximum of 15.8 cm with the level of 30.8 mmol L–1 NaCl (Figure 2A). In turn, the largest values of the radicle length were obtained at the 11.8 mmol L–1 NaCl level (Figure 2B). When the seeds were primed with water, the largest values of the shoot and radicle length were obtained at the levels of 7.8 and 11.8 mmol L–1 of NaCl, respectively (Figure 2A and 2B).
These results confirm the findings of Dantas et al. (2005) with their study on cowpea, [Vigna unguiculata L.], Memon et al. (2010) with their study on pak choi seedlings [Brassica campestris L.], and finally by Qados (2011) in their study on fava bean [Vicia faba L.] where they indicated that the use of low sodium chloride concentrations led to increases in plants lengths, whereas higher concentrations caused inhibition. In general, may be inferred that, the elongation of the stem when treated with low concentrations of salts may induce osmotic adjustment activity in the plants which may improve growth. On the other hand, excessive salt concentrations reduce the solution water potential, causing toxic effects and injuries and disorders in the metabolism of plants (Munns and Tester, 2008). Under high salinity an irreversible impairment of the photosynthetic apparatus, associated with a reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity, occurs when the stress is prolonged, and salt continues to accumulate in the leaves (Zhu, 2001). On the other hand, the noticed decrease in the length of the stem, also due to treatment with NaCl solution, could be due to the negative effect of this salt on the changes in enzyme activity (that subsequently affects protein synthesis), and also the decrease in the level of carbohydrates and growth hormones, both of which can lead to inhibition of the growth (Mazher et al., 2007).
The accumulation of shoot dry matter of wheat was reduced linearly with increasing salinity levels up to 100 mmol L–1 NaCl in both priming treatments (water or KNO3), however, this reduction was more pronounced in seeds subjected to water priming (control) (Figure 2C). At 100 mmol L–1 NaCl the shoot dry matter was 5.94 mg seedling–1 in seeds primed with water (control), and was significantly greater (8.41 mg seedling–1) when seeds were submitted to KNO3 priming. These results indicate that the KNO3 priming improved the initial growth of wheat shoots, and confirm the findings of Mohammadi (2009) with their study soybean [Glycine max (L.) Merrill.], who verified that the KNO3 priming led to increase of 58.1% in dry matter of seedlings. The exogenous application of KNO3 can produce substances that release NO, and this molecule acts as a signaler in higher plants that this involved in the regulation of plant growth and development, defense against pathogen and responses to abiotic stress (Sanz et al., 2015).
The lower accumulation of shoot dry matter due to salinity was caused by low external water potential, ion imbalance and specific ion toxicity (Feijão et al., 2011). One of the initial effects of salt stress on plant is the reduction of growth rate and dry matter accumulation. Oliveira et al. (2016) reported that salinity decreases the growth and dry matter accumulation of potato plants, and the salt level of 100 mmol L–1 decreased the shoot dry matter at around of 75%. In soybean plants, Dolatabadian et al. (2011) reported that salinity stress significantly decreased shoot and root dry matter, plant height and leaf number per plant.
Radicle length of wheat seedlings from the seeds subjected to KNO3 priming decreased progressively with rise of salinity levels (Figure 2B). The exposure of wheat seedlings to 100 mmol L–1 NaCl reduced the radicle length in 68% (from 17.9 to 5.7 cm) compared to the NaCl-free treatment, whereas accumulation of root dry matter was not significantly affected by salinity (Figure 2D). These results indicate that increasing the salt concentrations in the solution resulted in thicker roots. An early plant response to excess salts is the inhibition of expansion growth in leaves and roots. Furthermore, the salt stress is known to induce changes in root diameter. Increased root diameters under salinity have been found in a range of plant types, such as barley (Hordeum spp.), cotton (Gossypium hirsutum), Volkameriano lemmon (Citrus volkameriana), and lozina green (Tessaria absinthioides) (Huang and Redmann, 1995, Reinhardt and Rost, 1995; Degano, 1999; Rewald et al., 2012). The increased root diameter might be caused by succulence of the cortex. Succulence is an anatomical adaptation, which, by increasing the volume of vacuoles, permits the accumulation of larger amounts of water and dissolved ions in leaves, shoots and roots (Munns and Tester, 2008). Under the same NaCl level, the root succulence of two species of oleaster (Elaeagnus moorcroftii and E. oxycarpa) was found to be even slightly higher than leaf succulence (Wang et al., 2010). Indeed, increased root succulence has been shown to be a mechanism of adaptation and tolerance of plants to saline conditions (Degano, 1999). Besides increasing storage capacities, thicker roots are favorable to overcome increased soil strength, as prevails in some saline soils, and might have reduced maintenance respiration and turnover rates, resulting in reduced carbon costs below ground (Rewald et al., 2012).
The total dry matter of wheat seedlings was significantly affected by salt stress induced by NaCl solutions (Figure 3A). The inhibiting action of salt stress on plant growth was increased with the rise of salinity levels, and the exposure of seeds to 100 mmol L–1 NaCl reduced the accumulation of total dry matter in 24% and 14% compared to the NaCl-free treatment, respectively, for the seeds primed with water or KNO3.
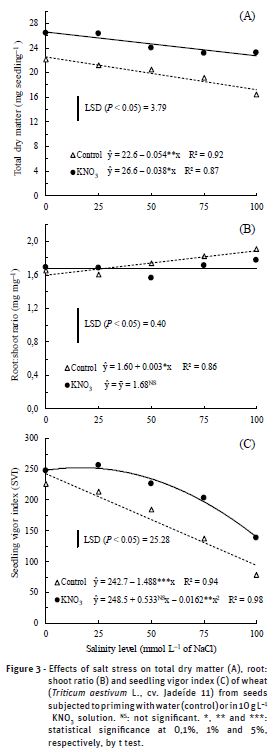
Root: shoot ratio is one of several ratios, which give estimates of dry matter partitioning into root and shoot of plants, and it is a good indicator for abiotic stress effects on root and shoot dry matter (Boutraa et al., 2010). The results showed that the root:shoot ratio of wheat seedlings from the seeds primed with water was increased with the rise of salinity levels (Figure 3B). The exposure of seedlings to 100 mmol L–1 NaCl increased the root:shoot ratio in 1.9% (from 1.60 to 1.63 mg mg–1) compared to the NaCl-free treatment. This suggests that shoot growth was affected more than the root system under salt stress. Such increase in root:shoot ratio indicates that the proportion of dry matter allocated to shoots was decreased compared to the roots. Studies have shown that shoot is more likely to be affected by saline stress than other traits, as reported by Wang et al. (2015) for cucumber plants. Assimilate partitioning is a complicated process that can be controlled simultaneously by sources and sinks. In general, plants exposed to high salt levels often partition photosynthate occurs preferentially to the roots, thereby maintaining a balance between processes required in roots (e.g.; water and nutrient uptake) and those required in shoots (e.g., photosynthesis). In turn, when the seeds were submitted to KNO3 priming, the root:shoot ratio was not significantly affected by saline stress induced by NaCl solutions (Figure 3B). These results indicate that the KNO3 priming improves the dry matter partitioning into root and shoot of wheat seedlings.
The vigor index of wheat seedlings was drastically reduced with the rise of salinity levels (Figure 3C). When the seeds were primed with KNO3, the largest values of the seedling vigor index were obtained at the 16 mmol L–1 NaCl level, whereas higher concentrations caused reduction in the seedling vigor index. In turn, when the seeds were primed with water, the vigor index decreased linearly with increasing salinity levels up to 100 mmol L–1 NaCl (Figure 3C). The seedling vigor index has been used as a tolerance index to evaluate the effect of salt stress on seedling growth (Ashkan and Jalal, 2013). Seedling vigor is a measure of the extent of damage that accumulates as viability declines, and the damage accumulates in seeds until the seeds are unable to germinate and eventually die (Marcos-Filho, 2005). The lower seedling vigor index obtained with increased salinity level was due to the salt stress inhibit the initial growth of plants, especially of the shoots. The reduction in vigor index of seedlings under water restriction conditions is commonly reported by other research (Zhang et al., 2007; Ashkan and Jalal, 2013; Singh et al., 2015; Liu et al., 2015).
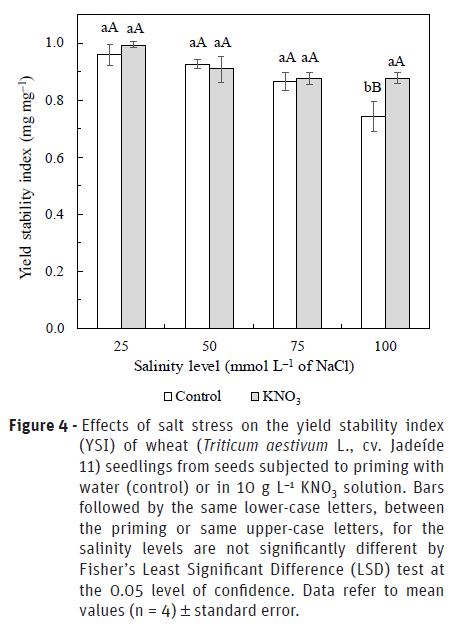
The yield stability index of wheat seedlings ranged from 0.96 to 0.74 and 0.99 to 0.88 for the seeds submitted to priming with water or KNO3, respectively (Figure 4). The yield stability index was suggested by Bouslama and Schapaugh (1984) to evaluation the stability of crops or genotypes in the both stress and non-stress conditions and has been considered a good salt tolerance index. When the yield stability index in response to addition of 25, 50, 75 and 100 mmol L–1 NaCl is greater than 0.95; 0.85; 0.65 and 0.50, respectively, the plant species is classified as moderately tolerant to salt stress (Mass, 1986). Therefore, results presented here suggest that wheat cultivar used in this study is a moderately tolerant genotype to the negative effects of high salt levels during the stage of seed germination and seedling establishment, confirming the results reported by Maas and Hoffman (1977). These authors pointed out that, in the field, where the salinity rises to 100 mmol L−1 NaCl (about 10 dS m−1), rice (Oryza sativa) will die before maturity, while wheat will produce a reduced yield. Singh et al. (2015) found that the salinity significantly reduced the yield of some wheat genotypes while some were found tolerant to stress indicating sufficient genetic variability for salinity tolerance. Therefore, the growing of a wheat cultivar with greater tolerance to high salt levels could improve the stability of crop grain yield under saline conditions.
CONCLUSIONS
Wheat seeds primed with potassium nitrate showed improved germination performance, early growth and vigor index of wheat seedlings in salt stress conditions, indicating that the deleterious effects of salinity can be reversed with the potassium nitrate priming.
Low salt concentrations may induce osmotic adjustment activity in the wheat plants and lead to increases in shoot and root length of wheat seedlings, whereas higher concentrations cause severe inhibition of plant growth.
The ‶Jadeíde 11″ wheat cultivar is a moderately tolerant genotype to salt stress during the seedling establishment stage by presenting yield stability index greater than 0.74 until the level of 100 mmol L–1 of NaCl.
References
Ahmadvand, G.; Soleimani, F.; Saadatian, B. and Pouya, M. (2012) - Effect of seed priming with potassium nitrate on germination and emergence traits of two soybean cultivars under salinity stress conditions.American-Eurasian Journal of Agricultural & Environmental Science, vol. 12, n. 6, p. 769-774. [ Links ]
Akram, M.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Iqbal, J. and Mohsan, M. (2010) - Screening for salt tolerance in maize (Zea mays L.) hybrids at an early seedling stage.Pakistan Journal of Botany, vol. 42, n. 1, p. 141-154. [ Links ]
Almansouri, M.; Kinet, J.M. and Lutts, S. (2001) - Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.).Plant and Soil, vol. 231, n. 2, p. 243-254. https://doi.org/10.1023/A:1010378409663 [ Links ]
Anosheh, H.P.; Sadeghi, H. and Emam, Y (2011) - Chemical priming with urea and KNO3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress.Journal of Crop Science and Biotechnology, vol. 14, n. 4, p. 289-295. https://doi.org/10.1007/s12892-011-0039-x [ Links ]
Ashkan, A. and Jalal, M. (2013) - Effects of salinity stress on seed germination and seedling vigor indices of two Halophytic Plant Species (Agropyron elongatum and A. pectiniforme).International Journal of Agriculture and Crop Sciences, vol. 5, n. 22, p. 2669-2676. [ Links ]
Bouslama, M. and Schapaugh, W.T. (1984) - Stress tolerance in soybean. Part 1: evaluation of three screening techniques for heat and drought tolerance.Crop Science, vol. 24, n. 5, p. 933-937. https://doi.org/10.2135/cropsci1984.0011183X002400050026x [ Links ]
Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.A. and Alhejeli, A.M. (2010) - Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia.Journal of Taibah University for Science, vol. 3, p. 39-48. https://doi.org/10.1016/S1658-3655(12)60019-3 [ Links ]
Brunes, A.P.; Fonseca, D.A.R.; Rufino, C.A.; Tavares, L.C.; Tunes, L.M. and Villela, F.A. (2013) - Crescimento de plântulas de aveia branca submetidas ao estresse salino.Semina: Ciências Agrárias, vol. 34, n. 6, p. 3455-3462. http://dx.doi.org/10.5433/1679-0359.2013v34n6Supl1p3455 [ Links ]
Chaves, L.C.G.; Andrade, E.M.; Crisostomo, L.A.; Ness, R.L.L. and Lopes, J.F.B. (2006) - Risk of degradation in irrigated soil at the distrito de irrigação do perímetro Araras Norte, Ceará, Brazil.Revista de Ciência Agronômica, vol. 37, n. 3, p. 293-299. [ Links ]
Dantas, B.F.; Ribeiro, L. and Aragão, C.A. (2005) - Physiological response of cowpea seeds to salinity stress.Revista Brasileira de Sementes, vol. 27, n. 1, p. 144–148. http://dx.doi.org/10.1590/S0101-31222005000100018 [ Links ]
Degano, C.A.M. (1999) - Respuestas morfológicas y anatómicas de Tessaria absinthioides (Hook. et Arn.) DC. a la salinidad. Revista Brasileira de Botânica, vol. 22, n. 3, p. 357-363. http://dx.doi.org/10.1590/S0100-84041999000300002 [ Links ]
Dolatabadian, A.; Modarressanavy, S.A.M. and Ghanati, F. (2011) - Effect of salinity on growth, xylem structure and anatomical characteristics of soybean.Notulae Scientia Biologicae, vol. 3, n. 1, p. 41–45. http://dx.doi.org/10.15835/nsb315627 [ Links ]
Feijão, A.R.; Silva, J.C.B.; Marques, E.C.; Prisco, J.T. and Gomes-Filho, E. (2011) - Efeito da nutrição de nitrato na tolerância de plantas de sorgo sudão à salinidade.Revista Ciência Agronômica, vol. 42, n. 3, p. 675-683. [ Links ]
Ferreira, D.F. (2011) - Sisvar: um sistema computacional de análise estatística. Ciência e Agrotecnologia, vol. 35, n. 6, p. 1039-1042. http://dx.doi.org/10.1590/S1413-70542011000600001 [ Links ]
Fuller, M.P.; Hamza, J.H.; Rihan, H.Z. and Al-Issawi, M. (2012) - Germination of primed seed under NaCl stress in wheat.International Scholarly Research Network Botany, vol. 2012, art 167804. http://dx.doi.org/10.5402/2012/167804 [ Links ]
Gordin, C.R.B.; Scalon, S.P.Q. and Masetto, T.E. (2015) - Disponibilidade hídrica do substrato e teor de água da semente na germinação de niger.Pesquisa Agropecuária Tropical, vol. 45, n. 3, p. 312-318. [ Links ]
Huang, J. and Redmann, R.E. (1995) - Salt tolerance of Hordeum and Brassica species during germination and early seedling growth.Canadian Journal of Plant Science, vol. 75, n. 4, p. 815-819. https://doi.org/10.4141/cjps95-137 [ Links ]
Kaiser, I.S.; Machado, L.C.; Lopes, J.C. and Mengarda, L.H.G. (2016) - Efeito de liberadores de óxido nítrico na qualidade fisiológica de sementes de repolho sob salinidade.Revista Ceres, vol. 63, n. 1, p. 39-45. http://dx.doi.org/10.1590/0034-737X201663010006 [ Links ]
Kaya, C.; Ashraf, M.; Dikilitas, M. and Tuna, A.L. (2013) - Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients – A field trial.Australian Journal of Crop Science, vol. 7, n. 2, p. 249-254. [ Links ]
Kaya, M.D.; Okçu G.; Atak M.; Cikili Y. and Kolsarici Ö. (2006) - Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.).European Journal of Agronomy, vol. 24, n. 4, p. 291-295. https://doi.org/10.1016/j.eja.2005.08.001 [ Links ]
Kubala, S.; Garnczarska, M.; Wojtyla, L.; Clippe, A.; Kosmala, A.; Żmieńko, A.; Stanley Lutts, S. and Quinet, M. (2015) - Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach.Plant Science, vol. 231, p. 94-113. https://doi.org/10.1016/j.plantsci.2014.11.008 [ Links ]
Labouriau, L.G. (1983) - A germinação de sementes. Washington: Organização dos Estados Americanos, 174 p. [ Links ]
Lawles, K.; Raun, W.; Desta, K. and Freeman K. (2012) - Effect of delayed emergence on corn grain yields.Journal of Plant Nutrition, vol. 35, n. 3, p. 480-496. https://doi.org/10.1080/01904167.2012.639926 [ Links ]
Liu, M.; Li, M.; Liu, K. and Sui, N. (2015) - Effects of drought stress on seed germination and seedling growth of different maize varieties.Journal of Agricultural Science, vol. 7, n. 5, p. 231-240. http://dx.doi.org/10.5539/jas.v7n5p231 [ Links ]
Maas, E.V. and Hoffman, G.J. (1977) - Crop salt tolerance - current assessment.Journal of Irrigation and Drainage Engineering ASCE, vol. 103, n. 2, p. 115-134. [ Links ]
Maguire, J.D. (1962) - Speed of germination - aid in selection and evaluation for seedling emergence and vigor.Crop Science, vol. 2, n. 1, p. 176-177. [ Links ]
MAPA (2013) - Padrões para produção e comercialização de sementes de aveia (Avena sativa L.) e trigo (Triticum aestivum L.). Instrução Normativa no 45, de 17 de setembro de 2013. Diário Oficial da União, sec.1, de 20/09/2013. Ministério da Agricultura, Pecuária e Abastecimento.
Marcos-Filho, J. (2005) - Fisiologia de sementes de plantas cultivadas. Piracicaba: FEALQ, 2005. 495 p. [ Links ]
Mass, E.V. (1986) - Salt tolerance of plants.Applied Agricultural Research, vol. 1, n. 1, p. 12-26. [ Links ]
Mazher, A.M.A.; El-Quesni, E.M.F. and Farahat, M.M. (2007) - Responses of ornamental and woody trees to salinity.World Journal of Agricultural Sciences, vol. 3, n. 3, p. 386–395. [ Links ]
Memon, S.A.; Hou, X. and Wang, L.J. (2010) - Morphological analysis of salt stress response of pak Choi.Electronic Journal of Environmental, Agricultural and Food Chemistry, vol. 9, n. 1, p. 248–254. [ Links ]
Meneses, C.H.S.G.; Bruno, R.L.A.; Fernandes, P.D.; Pereira, W.E.; Lima, L.H.G.M.; Lima, M.M.A. and Vidal, M.S. (2011) - Germination of cotton cultivar seeds under water stress induced by polyethyleneglycol-6000.Scientia Agricola, vol. 68, n. 2, p. 131-138. http://dx.doi.org/10.1590/S0103-90162011000200001 [ Links ]
Mohammadi, G.R. (2009) - The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.).American-Eurasian Journal of Agriculture & Environmental Science, vol. 5, n. 3, p. 322-326. [ Links ]
Munns, R. and Tester, M. (2008) - Mechanisms of salinity tolerance.Annual Review of Plant Biology, vol. 59, p. 651-681. https://doi.org/10.1146/annurev.arplant.59.032607.092911 [ Links ]
Oliveira, A.B and Gomes-Filho, E. (2009) - Germinação e vigor de sementes de sorgo forrageiro sob estresse hídrico e salino. Revista Brasileira de Sementes, vol. 31, n. 3, p. 48-56. http://dx.doi.org/10.1590/S0101-31222009000300005 [ Links ]
Oliveira, C.P.; Douradinho, G.Z.; Botolazzo, G. and Steiner, F. (2016) - Initial sprout growth of potato seed minitubers under salt stress.Revista de Agricultura Neotropical, vol. 3, n. 1, p. 7-11. [ Links ]
Oliveira, C.E.S. and Steiner, F. (2017) - Potassium nitrate priming to mitigate the salt stress on cucumber seedlings.Scientia Agraria Paranaensis, vol. 16, n. 4, p. 454-462. [ Links ]
Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P. and Sharma, K.K. (2017) - Nitric oxide (NO) in plant heat stress tolerance: current knowledge and perspectives. Frontiers in Plant Science, vol. 8, art. 1582. http://doi.org/10.3389/fpls.2017.01582 [ Links ]
Parida, A.K. and Das, A.B. (2005) - Salt tolerance and salinity effects on plants: a review.Ecotoxicology and Environment Safety, vol. 60, n. 3, p. 324-349. https://doi.org/10.1016/j.ecoenv.2004.06.010 [ Links ]
Pereira, B.L.C.; Borges, E.E.L.; Oliveira, A.C.; Leite, H.G. and Gonçalves, J.F.C. (2010) - Influência do óxido nítrico na germinação de sementes de Plathymenia reticulata Benth com baixo vigor.Scientia Forestalis, vol. 38, n. 88, p. 629-636. [ Links ]
Qados, A.M.S.A. (2011) - Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.).Journal of the Saudi Society of Agricultural Sciences, vol. 10, n. 1, p. 7–15. https://doi.org/10.1016/j.jssas.2010.06.002 [ Links ]
Reinhardt, D.H. and Rost, T.L. (1995) - Primary and lateral root development of dark- and light- grown cotton seedlings under salinity stress.Botanica Acta, vol. 108, n. 5, p. 457-465. https://doi.org/10.1111/j.1438-8677.1995.tb00521.x [ Links ]
Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J.E. and Rachmilevitch, S. (2012) -Phenotypic plasticity and water flux rates of Citrus root orders under salinity.Journal of Experimental Botany, vol. 63, n. 7, p. 2717-2727. https://dx.doi.org/10.1093%2Fjxb%2Ferr457 [ Links ]
Richards, L.A. (1954) - Diagnosis and improvement of saline and alkali soils. Washington DC: United States Department of Agriculture. 160 p. [ Links ]
Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos M. and Lorenzo, O. (2015) - Nitric oxide (NO) and phytohormones crosstalk during early plant development. Journal of Experimental Botany, vol. 66, n. 10, p. 2857-2868. https://doi.org/10.1093/jxb/erv213 [ Links ]
Singh, S.; Sengar, R.S.; Kulshreshtha, N.; Datta, D.; Tomar, R.S.; Rao, V.P.; Garg, D. and Ojha, A. (2015) - Assessment of multiple tolerance indices for salinity stress in bread wheat (Triticum aestivum L.). Journal of Agricultural Science, vol. 7, n. 3, p. 49-57. http://dx.doi.org/10.5539/jas.v7n3p49 [ Links ]
Wang, S.; Liu, P.; Chen, D.; Yin, L.; Li, H. and Deng, X. (2015) - Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber.Frontiers in Plant Science, vol. 6, art. 759. https://dx.doi.org/10.3389%2Ffpls.2015.00759 [ Links ]
Wang, Y.; Zhang, X.M.; Cao, Z.H.; Liu, W. and Wang, Q. (2010) - Effect of salt stress on seedlings biomass of two varieties of Elaeagnus spp.China Forestry Science and Technology, vol. 24, p. 25-28. [ Links ]
Zanotti, R.F.; Lopes, J.C.; Motta, L.B.; Freitas, A.R. and Mengarda, L.H.G. (2013) - Tolerance induction to saline stress in papaya seeds treated with potassium nitrate and sildenafil citrate.Semina: Ciências Agrárias, vol. 34, n. 6, p. 3669-3674. [ Links ]
Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J. and Allen, K. (2007) - Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Australian Journal of Agricultural Research, vol. 58, n. 8, p. 811–815. https://doi.org/10.1071/AR06253 [ Links ]
Zhu, J.K. (2001) - Plant salt tolerance.Trends in Plant Science, vol. 6, n. 2, p. 66-71. https://doi.org/10.1016/S1360-1385(00)01838-0 [ Links ]
Received/recebido: 2016.10.15
Received in revised form/recebido em versão revista: 2017.12.22
Accepted/aceite: 2018.06.22














