Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.41 no.spe Lisboa dez. 2018
https://doi.org/10.19084/RCA.17063
ARTIGO
Diversity of fungal endophytic community in Quercus suber L. under different climate scenarios
Diversidade da comunidade de fungos endofíticos em Quercus suber em diferentes cenários climáticos
Daniela Costa1, Rui M. Tavares1, Paula Baptista2 and Teresa Lino-Neto1*
1 BioSystems & Integrative Sciences Institute (BioISI), Plant Functional Biology Centre, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. 2CIMO/School of Agriculture, Polytechnic Institute of Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal
(*E-mail: tlneto@bio.uminho.pt)
ABSTRACT
Cork oak (Quercus suber L.) is an evergreen oak tree species, typically found throughout the Mediterranean Basin, which presents a great ecological and economic importance for Portugal. An increase of cork oak decline due to biotic and abiotic stresses has been reported, which could damage the ecosystem and lower cork production. The attack of some fungal pathogens seems to increase when trees are under several environmental stresses, such as increased temperatures or drought. In this work, fungal endophytic species of cork oak were collected from forest stands in different sites of Portugal. The community of fungal endophytes of young and old twigs was studied by obtaining fungal isolates from plant material and grouping them into morphotypes. This study allowed the comparison of fungal endophytic communities living in cork oak trees from different forests, displaying distinct climates and water availability levels. The results revealed that endophyte communities are better discriminated when considering different plant tissues than cork oak stand location or climate.
Keywords: cork oak, endophyte, endophytic community, fungi
RESUMO
O sobreiro (Quercus suber L.) é uma espécie arbórea de folha perene, encontrada tipicamente na Bacia do Mediterrâneo, que apresenta uma elevada importância ecológica e económica para Portugal. Um aumento no declínio do sobreiro tem sido associado a situações de stresse biótico e abiótico, o qual resulta em danos no ecossistema e diminuição da produção de cortiça. A infeção por fungos patogénicos parece aumentar quando os sobreiros se encontram sob condições ambientais promotoras de stresse, como temperatura elevada e secura. Neste trabalho, as espécies fúngicas endófitas de sobreiro foram colhidas a partir de exemplares em diferentes locais de Portugal. A comunidade de fungos endófitos de ramos com diferentes idades foi estudada pela obtenção de isolados a partir deste material vegetal, e pelo agrupamento dos isolados em morfótipos. Este estudo permitiu a comparação das comunidades fúngicas endófitas em diferentes povoamentos florestais, sujeitos a condições climáticas distintas e a diferentes níveis de disponibilidade de água. Os resultados revelam que as comunidades endófitas são distintas entre os diferentes tecidos vegetais, sendo mais semelhantes entre os locais das florestas ou clima.
Palavras-chave: comunidade endofítica, endófito, fungos, sobreiro
INTRODUCTION
Cork oak (Quercus suber L.) is an evergreen tree species characterized by slow growing rates and long-lived exemplars (Gil & Varela, 2008). Cork oak forests occupy more than 2 million ha worldwide representing a great socio-economic and ecological importance, in particular for the Mediterranean Basin. The Iberian Peninsula has the largest cork oak forests with an area of 1,290,170 ha (34.9% in Portugal and 27.9% in Spain) (Allard et al., 2013). One of the most interesting features of Q. suber is the production of cork, a thick bark that covers the trunk and branches, which is naturally produced every year by cork oak tree (Pereira, 2007). Cork has sealing and insulating characteristics, being mainly used in the production of wine bottle stoppers but also to produce pavements and insulating materials (Allard et al., 2013). The Iberian Peninsula is responsible for 80% of annual cork production worldwide, Portugal contributing with almost 50% of total production. Moreover, Portugal has an important industry for raw cork processing, which is reflected by a share of 62.7% from the 1,430.8 million euros generated by cork exportations worldwide (APCOR, 2016).
Climate change projections indicate an increase in annual temperature and a decrease in annual precipitation (Giorgi & Lionello, 2008), also predicting that the Mediterranean region will be one of the most affected regions worldwide (Giorgi, 2006). Cork oak growth and cork quality can be severely affected under drought conditions, which could also promote an increase of declined cork oaks (Costa et al., 2010; Acácio et al., 2017). The increase of abiotic stress in cork oak trees can also potentiate infections of microorganisms with opportunistic behaviour, which would be reflected by an increase of cork oak diseases (Moricca et al., 2016). Endophytic fungi, as Diplodia corticola and Biscogniauxia mediterranea, have been increasingly reported as causing decline in cork oak trees (Franceschini et al., 2005; Henriques et al., 2012). The incidence of these endophytic fungi is also increased in cork oak trees under environmental stresses, such as drought (Linaldeddu et al., 2011). This work pretends to determine the diversity of endophytic communities associated with cork oak tree branches and detect potential fluctuations of endophytes on organ tissues or locations with different climates.
MATERIAL AND METHODS
Biological material and sample collection
Cork oak tree samples were collected from six locations in continental Portugal (Table 1, Figure 1) selected based on previous available information (Varela and Eriksson, 1995), and Mediterranean climate classification (Rego and Rocha, 2014), as determined by the climatic parameter of Emberger (Q, Emberger, 1930). Annual precipitation occurring during the previous year of sampling (2016) ranged between 1704.2 mm (National Park of Peneda-Gerês, PG) and 510.6 mm (Herdade da Contenda, HC), corresponding to Emberger indexes of 186.6 and 43.5, respectively. Two independent stands were sampled from these extreme sites (PG-ER and PG-RC, from PG location; HC-CT and HC-MA, from HC location). The other stands presented intermediate values for annual precipitation (870.5-751.9) and Emberger indexes (102.7 to 77.5). From each sampling site, five to seven trees were selected, from which five to seven young branch samples were taken. The collection of biological material was performed between April and October of 2017.
Plant tissue sterilization and plating
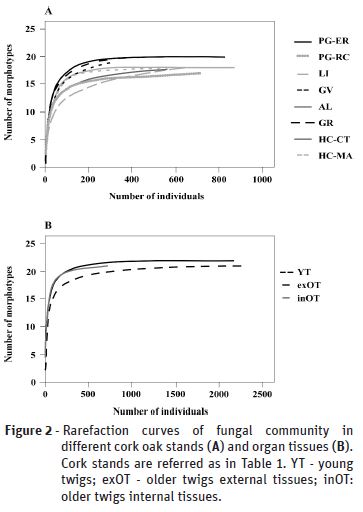
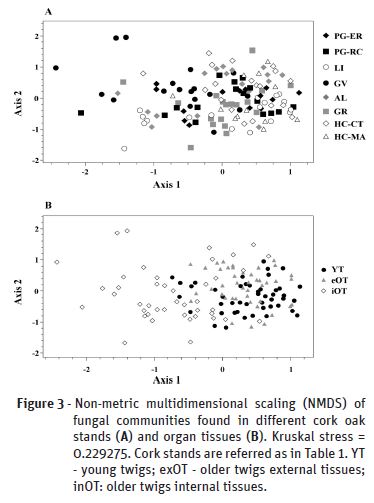
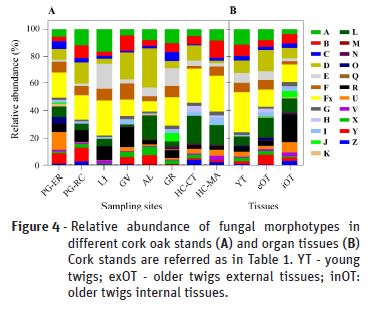
From each branch, young twigs (with a diameter up to 0.5 cm: YT) and older twigs (with a diameter up to 1.5 cm, OT) were thoroughly washed with tap water and surface-sterilized, in order to remove the epiphytes. The sterilization process was optimized from Martins et al. (2016) and was performed by washing the plant material with deionized water, followed by the sequential immersion in ethanol 70% (v/v) for 2 minutes, bleach (3-5% chlorine) for 6 minutes, ethanol 70% (v/v) for 1 minute, followed by three washes in sterile deionized water (1 minute each) and drying. Water from the last immersion (10 µl) was used as control after being plated onto Potato Dextrose Agar (PDA) medium. Younger twigs were cut into 4-5 cm segments, which were immediately transferred onto PDA medium (5 segments/plate) and incubated at room temperature in dark. Older twigs were longitudinally cut to separate the most external region (exOT), including all living tissues, from the internal tissue (inOT) composed by xylem. Five fragments of each were transferred onto PDA (5 fragments/plate) and incubated as previously referred. From each condition (stand/organ), 3 replicates were done. Altogether, for YT, exOT and inOT, 9435 segments/fragments were assayed (8 stands x 5-7 trees x 5-7 branches x 5 segments/fragments x 3 replicates). The outgrowing fungi were assigned as endophytes and sub-cultured to fresh PDA medium to obtain pure cultures. Identification of fungal morphotypes and fungal occurrence Endophytic fungi were grouped into morphotypes based on their cultural features, such as color, shape, elevation, and margins (Table 2). Fungal occurrence in each organ and cork oak stand was measured by determination of frequency of colonization (FC) and relative abundance (RA). FC was calculated using the number of colonized segments/fragments divided by the total number of segments/fragments surveyed for each organ/stand. RA was measured dividing the total number of fungi that colonized each organ/stand by the total number of colonizing fungi. RA of each fungal morphotype was also determined for each organ/stand by dividing the number of fungal isolates of each morphotype in that organ/stand by the total number of fungal isolates. Variation of RA for each fungal morphotype was plotted using GraphPad Prism 6 (GraphPad Software, 1994). Morphotype diversity and statistical analysis Fungal morphotype diversity was compared among stands and organs using diversity indexes and rarefaction curves that were computed using Species Diversity and Richness 4.1.2 (Seaby & Henderson, 2006). The diversity indexes of Shannon-Wiener’s (H’) and Simpson’s (D) take into account both the number of morphotypes and the proportion in which they are represented (Hughes & Bohannan, 2004). For understanding how morphotype community composition changes between cork oak stands, non-metric multidimensional scaling (NMDS) was computed by Community Analysis Package 5 (Seaby & Henderson, 2014) with similarity measures based in Bray Curtis coefficient. RESULTS AND DISCUSSION Fungal colonization of cork oak Cork oak stands of six different regions of Portugal were surveyed for fungal endophytes residing on their twigs. All fungi that were outgrowing from tested segments/fragments were registered as endophytes and grouped into different fungal morphotypes, according to their cultural features (Table 2). In total, 46 cork oak trees were sampled, presenting the assayed segments/fragments (9450) a global frequency of colonization of 27.42% (Table 3), resulting in 5182 endophyte counts. The highest frequency of colonization was found in PG-ER stand (37.30%), while the lowest was in GV (12.61%). The relative abundance of fungal isolates followed this trend, presenting the PG-ER stand (15.98%) a higher RA than GV that presented the lowest RA (5.96%) of all stands. However, this tendency was not always verified since HC-CT stand presented the highest RA (16.92%), but a lower FC (32.72%) than PG-ER. This result is due to the presence of more than a single fungi outgrowing from the same segment/fragment, while many segment/fragments did not exhibit fungal growth. Regarding the differential colonization of tissue organs, the external older twigs exhibited the highest colonization and fungal abundance rates, whereas the internal tissues exhibited the lowest. This pattern was constant for all cork oak stands, although YT and exOT tissues compete for the highest colonized tissue and highest fungal abundance. For example, in contrast with other forest stands, PG-ER, LI and AL stands displayed higher colonization and fungal abundance in young than in older twigs (external regions). As expected, these results reveal that internal tissues (composed by inactive xylem) have fewer endophytes than external tissues. Besides being more exposed to the environment, external tissues comprise other tissues that could be the habitat of endophytes. Indeed, low frequency of endophytes in sapwood has been reported in Azadirachta indica (Verma et al., 2007) and in different Chilean trees (Oses et al., 2008). Furthermore, the colonization frequency and abundance of fungal endophytes have been described to be distinct in organ tissues from different species (Naik et al., 2009; Martins et al., 2016; Gomes et al., 2018). Fungal diversity and community similarity Cultural features of the isolates allowed the distinction of 23 fungal morphotypes (Table 2). The number of identified morphotypes varied when considering different cork oak stands (non-significant differences, Table 3). The diversity of endophytic fungal community was compared using rarefaction curves (Figure 2) and computation of diversity indexes (Table 3). Rarefaction curves revealed a more diverse community in cork oak stand PG-ER and GR, while PG-RC stand presented the less diverse community, still requiring a sampling effort for representing well whole endophyte community of that cork oak forest (Figure 2A). In contrast, PG-ER, GR, HC-MA and HC-CT curves reached a plateau, suggesting that the sampling effort was enough to represent these communities. According to these results, PG-ER and GR were the stands with the highest diversity parameters (H’ and D, Table 3), which were followed by GV forest. The cork oak stand LI presented the less diverse community. Once more, there was not a clear distinction among endophyte communities from the most humid regions (PG-ER, PG-RC and LI) and most arid regions (GR, HC-CT and HC-MA). Rarefaction curves from organ tissues revealed that any endophytic community was completely represented, being necessary more sample analysis to get the total richness on endophytes (Figure 2B). Nevertheless, cumulative curves revealed that exOT tissues comprised the most diverse community. Diversity indexes (H’ and D) also pointed exOT as comprising a high diverse endophyte community (Table 3). However, H’ and D indexes were not statistically different between exOT and inOT endophyte community, revealing YT community as the least diverse of all. Higher diversity of endophytes in older organs has been reported and could be explained by the age of the organ, as described in Coccoloba cereifera leaves (Sanchez-Azofeifa et al., 2012). Non-metric multidimensional scaling (NMDS) was performed for understanding fungal endophytic similarities among cork oak samples (Figure 3). As previously suggested, samples from different cork oak stands did not present any clustering according to their location or climate/water availability regions. Some dissimilarities were found for the inOT endophyte community, when compared to exOT or YT communities that clustered together. Fungal morphotype composition The relative abundance of each fungal morphotype in cork oak stands is variable, although there is a clear predominance of Fx morphotype in all stands, followed by D and L morphotypes (Figure 4A). In contrast, several morphotypes presented less than 1% abundance (J, K, M, N, O and Q). Twelve morphotypes were identified in all cork oak stands, while others were more specific from certain regions. For example, N morphotype was specific from southern regions, where water is less available. The influence of geographic location for endophytic community distribution was already described for Quercus ilex (Collado et al., 1999). In addition, fungal endophytes occurrence seems to be influenced by season as referred for the Indian medicinal plant Tinospora cordifolia (Mishra et al., 2012) or by precipitation as reported for Populus deltoides (Shakya et al., 2013) and Plumeria rubra (Suryanarayanan & Thennarasan, 2004). Regarding cork oak organs, there was a different pattern between tissues (Figure 4B). The dominant morphotype in YT tissues was Fx morphotype, while in exOT tissues were D and L morphotypes and in inOT tissues was R morphotype. This suggests that R fungal morphotype could be more associated with inactive xylem tissues present in inOT tissues. Indeed, fungal endophytic species can be found in some organs and be absent in others. For example, Chaetomium crispatum was found in twigs but not in the bark of Terminalia arjuna (Tejesvi et al., 2005) and Trichoderma koningiopsis was identified in sapwood but not in leaves of Hevea brasiliensis (Gazis & Chaverri, 2010). In addition, different endophytic fungal species were isolated from xylemic and phloemic tissues of Sophora tonkinensis roots (Yao et al., 2017). CONCLUSIONS In this work, the fungal endophytic community present on twigs from cork oaks of eight forest stands were studied. Although sampling stands were well distributed in Portugal, comprising more humid (PG-ER, PG-RC and LI) as well as more arid forest stands (GR, HC-CT and HC-MA), there was not a clear distinction among fungal endophyte community, as evaluated by cultural morphotypes. A difference was registered when considering the tissues evaluated (young and older twigs, including external and internal tissues). While internal tissues from older twigs, composed exclusively by xylem, presented a low colonization by endophytes but a rich and diverse community, young twigs were highly colonized by endophytes but present a less diverse endophyte community. These differences could be due to the exposition of young twigs to environmental conditions that promote fungal colonization. The fungal identification of morphotypes will help the elucidation of cork oak endophyte communities. REFERENCES Acácio, V.; Dias, F.S.; Catry, F.X.; Rocha, M. & Moreira, F. (2017) - Landscape dynamics in Mediterranean oak forests under global change: understanding the role of anthropogenic and environmental drivers across forest types. Global Change Biology, vol. 23, n. 3, p. 1199–1217. https://doi.org/10.1111/gcb.13487 [ Links ] Allard, G.; Berrahmouni, N.; Besacier, C. et al. (2013) - State of forest resources in the Mediterranean Region. In: Christophe, B; Valentina, G. & David, S.A. (Eds.) - State of Mediterranean Forests 2013, Ch. 2. Rome, FAO. <http://www.fao.org/docrep/017/i3226e/i3226e.pdf> [ Links ] APCOR (2016) - Cork Yearbook 2016. Available at: Portuguese Cork Association, Santa Maria de Lamas. <https://www.apcor.pt/en/portfolio-posts/apcor-year-book-2016> [ Links ]. Collado, J.; Platas, G.; González, I. & Peláez, F. (1999) - Geographical and seasonal influences on the distribution of fungal endophytes in Quercus ilex. The New Phytologist, vol. 144, n. 3, p. 525-532. https://doi.org/10.1046/j.1469-8137.1999.00533.x [ Links ] Costa, A.; Pereira, H. & Madeira, M. (2010) - Analysis of spatial patterns of oak decline in cork oak woodlands in Mediterranean conditions. Annals of Forest Science, vol. 67, n. 2, p. 204–204. https://doi.org/10.1051/forest/2009097 [ Links ] Emberger, L. (1930) - Sur une formule climatique applicable en géographie botanique. Comptes Rendus de l’Académie des Sciences, vol. 191, p. 389-390. Franceschini, A.; Linaldeddu, B.T. & Marras, F. (2005) - Occurrence and distribution of fungal endophytes in declining cork oak forests in Sardinia (Italy). IOBC/WPRS Bulletin, vol. 28, p. 67–74. [ Links ] Gazis, R. & Chaverri, P. (2010) - Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecology, vol. 3, n. 3, p. 240-254. https://doi.org/10.1016/j.funeco.2009.12.001 [ Links ] Gil, L. & Varela, M.C. (2008) - EUFORGEN Technical guidelines for genetic conservation and use for cork oak (Quercus suber). Biodiversity International, vol. 6. <https://www.bioversityinternational.org/fileadmin/_migrated/uploads/tx_news/Cork_oak__Quercus_suber__1323.pdf> [ Links ] Giorgi, F. (2006) - Climate change hot-spots. Geophysical Research Letters, vol. 33, n. 8, art. L08707. https://doi.org/10.1029/2006GL025734 [ Links ] Giorgi, F. & Lionello, P. (2008) - Climate change projections for the Mediterranean region. Global and Planetary Change, vol. 63, n. 2-3, p. 90–104. https://doi.org/10.1016/j.gloplacha.2007.09.005 [ Links ] Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T. & Baptista, P. (2018) - Endophytic and Epiphytic Phyllosphere Fungal Communities Are Shaped by Different Environmental Factors in a Mediterranean Ecosystem. Microbial Ecology, vol. 76, n. 3, p. 668-679. https://doi.org/10.1007/s00248-018-1161-9 [ Links ] Graphpad Software (1994) - Graphpad software. San Diego, CA, USA. [ Links ] Henriques, J.; Inácio, M.L.; Lima, A. & Sousa, E. (2012) - New outbreaks of charcoal canker on young cork oak trees in Portugal. IOBC/WPRS Bulletin, vol. 76, p. 85–88. [ Links ] Hughes, J.B. & Bohannan, B.J.M. (2004) - Application of ecological diversity statistics in microbial ecology. In: Molecular microbial ecology manual. vol. 2. Kluwer Academic Publishers, Dordrecht, the Netherlands. [ Links ] Linaldeddu, B.T.; Sirca, C., Spano, D. & Franceschini, A. (2011) - Variation of endophytic cork oak-associated fungal communities in relation to plant health and water stress. Forest Pathology, vol. 41, n. 3, p. 193–201. https://doi.org/10.1111/j.1439-0329.2010.00652.x [ Links ] Martins, F.; Pereira, J.A.; Bota, P.; Bento, A. & Baptista, P. (2016) - Fungal endophyte communities in above- and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal Ecology, vol. 20, p. 193–201. https://doi.org/10.1016/j.funeco.2016.01.005 [ Links ] Mishra, A.; Gond, S.K.; Kumar, A.; Sharma, V.K., Verma, S.K.; Kharwar, R.N. & Sieber, T.N. (2012) - Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microbial Ecology, vol. 64, n. 2, p. 388-398. https://doi.org/10.1007/s00248-012-0029-7 [ Links ] Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A. & Ragazzi, A. (2016) - Endemic and emerging pathogens threatening cork oak trees: Management options for conserving a unique forest ecosystem. Plant Disease, vol. 100, n. 11, p. 2184–2193. https://doi.org/10.1094/PDIS-03-16-0408-FE [ Links ] Naik, B.S.; Shashikala, J. & Krishnamurthy, Y.L. (2009) - Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiological Research, vol. 164, n. 3, p. 290-296. https://doi.org/10.1016/j.micres.2006.12.003 [ Links ] Oses, R.; Valenzuela, S.; Freer, J.; Sanfuentes E. & Rodriguez, J. (2008) - Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Diversity, vol. 33, p. 77–86. [ Links ] Pereira, H. (2007) - Cork: Biology, Production and Uses. Amsterdam, Netherlands: Elsevier. [ Links ] Rego, F.C. & Rocha, M.S. (2014) - Climatic patterns in the Mediterranean region. Ecologia Mediterranea, vol. 40, n. 1, p. 49-60. [ Links ] Sanchez-Azofeifa, A.; Oki, Y.; Fernandes, G.W.; Ball, R.A. & Gamon, J. (2012) - Relationships between endophyte diversity and leaf optical properties. Trees, vol. 26, n. 2, p. 291-299. https://doi.org/10.1007/s00468-011-0591-5 [ Links ] Seaby, R.M. & Henderson, P. A. (2006) - Species diversity and richness version 4. Pisces Conservation Ltd., Lymington, England. [ Links ] Seaby, R.M. & Henderson, P.A. (2014) - Community Analysis Package 5.0. [ Links ] Shakya, M.; Gottel, N.; Castro, H.; Yang, Z.K.; Gunter, L.; Labbé, J.; Muchero, W.; Bonito, G.; Vilgalys, R.; Tuskan, G. & Podar, M. (2013) - A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS One, vol. 8, art. e76382. https://doi.org/10.1371/journal.pone.0076382 [ Links ] Suryanarayanan, T.S. & Thennarasan, S. (2004) - Temporal variation in endophyte assemblages of Plumeria rubra leaves. Fungal Diversity, vol. 15, p. 197-204. [ Links ] Tejesvi, M.V.; Mahesh, B.; Nalini, M.S.; Prakash, H.S.; Kini, K.R.; Subbiah, V. & Shetty, H.S. (2005) - Endophytic fungal assemblages from inner bark and twig of Terminalia arjuna W. & A. (Combretaceae). World Journal of Microbiology and Biotechnology, vol. 21, n. 8-9, p. 1535-1540. http://dx.doi.org/10.1007/s11274-005-7579-5 [ Links ] Varela, M.C. & Eriksson, G. (1995) - Multipurpose gene conservation in Quercus suber- a Portuguese example. Silvae Genetica, vol. 44, p. 28-36. [ Links ] Verma, V.C.; Gond, S.K.; Kumar, A.; Kharwar, R.N. & Strobel, G. (2007) - The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachta indica A. Juss (Neem) from Varanasi (India). Microbial Ecology, vol. 54, n. 1, p. 119-125. https://doi.org/10.1007/s00248-006-9179-9 [ Links ] Yao, Y.Q.; Lan, F.; Qiao, Y.M.; Wei, J.G.; Huang, R.S. & Li, L.B. (2017) - Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: Diversity and biocontrol potential against phytopathogens. MicrobiologyOpen, vol. 6, n. 3, art. e00437. https://doi.org/10.1002/mbo3.437 [ Links ] ACKNOWLEDGEMENTS This work was supported by the European Regional Development Fund (ERDF), through the Operational Programme for Competitiveness and Internationalization (COMPETE 2020), under Portugal 2020, and by the Fundação para a Ciência e a Tecnologia – FCT (National Agency for Science and Technology) through national funds in the framework of the project POCI-01-0145-FEDER-0311332, and national funds by FCT under the projects PEst-OE/BIA/UI4046/2014 and UID/MULTI/04046/2013. D. Costa thanks FCT for PhD grant SFRH/BD/120516/2016. Received/recebido: 2018.01.20 Accepted/aceite: 2019.01.08