Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.42 no.2 Lisboa jun. 2019
https://doi.org/10.19084/rca.17172
ARTIGO
Microbial activity and population of a red-yellow podzolic soil under organic and conventional cultivation systems of Citrus sinensis (L.) Osbeck
Atividade e população microbiana de um podzólico vermelho amarelo sob sistemas de cultivo orgânico e convencional de Citrus sinensis (L.) Osbeck
João Manoel da Silva1,*, Crísea Cristina Nascimento de Cristo1, Yamina Coentro Montaldo1, Clayton dos Santos Silva1, Edinaldo de Oliveira Alves Sena2, Ricardo Brauer Vigoderis3, Geovanny Barroso4, José Soares Brito Neto1, José Ubaldo Lima de Oliveira1 and Tania Marta Carvalho dos Santos1
1Laboratório de Microbiologia, Centro de Ciências Agrárias Universidade Federal de Alagoas. BR 101, Rio Largo, Alagoas, Brasil. CEP 49100-000
2Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brasil
3Departamento de Agronomia, Universidade Federal Rural de Pernambuco, Unidade Acadêmica Garanhuns, Av. Bom Pastor, Garanhuns, Pernambuco
4Escola Superior de Agricultura "Luiz de Queiroz", Departamento de Entomologia e Acarologia. Avenida Pádua Dias, 11 - Cx. Postal 9, Brasil
(*E-mail: joao.manoel@iqb.ufal.br)
ABSTRACT
Fruticulture has relevant expression for worldwide plant production. With the expansion of agricultural areas, more attention is given to the soil preservation. Microbial activity is one of the parameters that together with the evaluation of microbial community provide data about soil biological quality. Therefore, the objective of this study was to evaluate the activity and microbial population of a red-yellow podzolic soil cultivated with Citrus sinensis under conventional and organic systems. Thus, soil samples were collected in two cultivated areas, under these both systems. A fraction of each soil sample was separated and autoclaved to determine the presence of thermoresistant microorganisms. The microbial activity was measured using the method of capturing CO2. The microbial population was estimated by selective culture medium, followed by counting of colony forming units. All data was submitted to analysis of variance and covariance (p≤0.05 and 0.01 respectively). It was observed that, initially, there was a difference in the microbial activity between the cultivation systems, and there was also a high bacterial population. Filamentous fungi and actinomycetes have distinct and peculiar behavior, which were function of the cultivation system and also of the soil samples heating.
Keywords: microbial diversity, soil quality, actinomycetes, fungus, bacteria
RESUMO
A fruticultura tem expressão relevante na produção mundial de plantas. Com a expansão das áreas agrícolas, mais atenção está sendo dada à preservação dos solos. A atividade microbiana é um dos parâmetros que, juntamente com a avaliação da comunidade microbiana, fornece dados sobre a qualidade biológica do solo. Assim, o objetivo deste trabalho foi avaliar a atividade e a população microbiana de um solo podzólico vermelho-amarelo cultivado com Citrus sinensis em sistemas convencional e orgânico. Para isso, as amostras de solo foram colhidas em duas áreas cultivadas, uma sob sistema convencional e outra sob sistema orgânico. Uma fração de cada amostra de solo foi separada e autoclavada para se determinar a presença de microrganismos termorresistentes. A atividade microbiana foi medida usando o método de captura de CO2. A população microbiana foi estimada por meio de cultivo seletivo, seguida pela contagem de unidades formadoras de colónias. Todos os dados foram submetidos a uma análise de variância e covariância (p≤0,05 e 0,01 respectivamente). Observou-se que, inicialmente, houve uma diferença na atividade microbiana entre os sistemas de cultivo, e também a presença de uma alta população bacteriana. Verificou-se ainda que os fungos filamentosos e actinomicetas têm comportamentos distintos e peculiares em função do sistema de cultivo e também do aquecimento das amostras de solo.
Palavras-chave: diversidade microbiana, qualidade do solo, actinomicetas, fungos, bactérias
INTRODUCTION
Brazilian fruit production is currently diversified when it comes to the cultivated fruit. Citriculture, in turn, had elevated the country to a privileged position, with Brazil being the world‘s largest producer of this modality (IBGE, 2017). However, several challenges are tackled daily to maintain the productive development of citrines.
For a long time, the world population has been worrying about food quality and safety, thinking about the next generations, which makes it possible to look for better quality foods, also considering the cultivation methods. In this respect, conventional and organic cultivation systems are conceived for citriculture.
The intensification of agricultural production influences an increase of the machines flow in the orchard, where they cause soil damage (Minatel et al., 2006), affecting their physical qualities, resulting in disturbances in the chemical and biological structure, affecting also the fertility. The soil is understood as a living compartment sheltering an infinity of organisms, which contribute directly and indirectly to the fertility, interacting also with the plants.
In conventional crops there is an increase in inputs, which affect the microbial structure of the soil (Sapp et al., 2015), for example through the application of fungicides (Balzergue et al., 2013), which are reported as directly affecting the soil microbiota populations (Zhou et al., 2011; Campos et al., 2015; Wang et al., 2016). In organic cultivation systems there are still some deficiencies regarding plant nutrition, which generates low productivity by productive units. However, it is already known that in these systems there are an interaction and diversity of microorganisms that are capable of increasing the production through microorganism/plant interaction, such as the presence of arbuscular mycorrhizal fungi (Wang et al., 2012).
There are many mechanisms for inferring about soil quality. Microbial respiration is one of these methods, and is used as a means of inferring microbial activity in crop systems (Barroso et al., 2012), as well as the microbial population (Montaldo et al., 2018). Thus, the objective of this study was to evaluate the respiratory activity and soil microbial population under cultivation of Citrus sinensis in conventional and organic systems.
MATERIAL AND METHODS
Experimental area description and soil sampling
The experiment was conducted in two areas of commercial cultivation of C. sinensis, one in organic cultivation system (which has organic production certification with the current legislation) and the other in conventional cultivation system (with use of agrochemicals and other inputs). The soil of the region is classified as red-yellow Podzolic (EMBRAPA, 2014). The climate is classified as tropical humid, which is characterized as ideal for the cultivation of fruit plants of tropical and semi-tropical climate.
Soil samples were collected, in both areas, at 20 cm depth from the soil surface, being 10 sub samples in each area. Finally, these sub samples were homogenized, resulting in two composite samples, which were identified and sent to the laboratory for analyzes.
From each soil sample (conventional and organic), half was separated and autoclaved for one cycle of 1h in order to estimate the presence of thermotolerant microorganisms.
Microbiological and chemical analysis
Samples were submitted to microbiological analysis by cultivation on selective and specific culture medium. For this, a serial decimal dilution of the soil samples was performed by diluting 1g of soil in erlenmeyer flasks containing 90mL of saline solution and the dilution was carried out until reaching the fraction 10-6. To determine the soil fungus population, the x10-4 dilution was used. Aliquots of 1mL of the dilution were inoculated into Petri dishes containing Martin medium (Martin, 1950), which is a selective medium for filamentous fungi and yeasts. The plates were incubated at room temperature for 7 days in the dark and then counting colony forming units per gram (CFU g-1) of soil was performed.
For the isolation of total bacteria and actinomycetes, the media Nutrient Agar (Fahy and Hayward, 1983) and Starch Casein (Kuster and Williams, 1964), respectively, were used. Likewise, the x10-6 dilution was used for both microorganisms. For total bacteria the Petri dishes were incubated for 3 days and 10 days for the actinomycetes. At the end of the incubation period, plates were submitted to the CFU counts. All the tests were performed with 6 replicates.
The chemical analyzes were carried out at the Soil Physics and Fertility Laboratory of the Federal University of Alagoas.
Microbial activity essay
In order to measure the soil microbial activity, the soil carbon capture method was calculated using the metabolic coefficient, estimated by the C-CO2 ratio of the basal microbial respiration and the C of the microbial biomass of the samples, according to Anderson and Domsch (1993).
Fractions of 150g of soil were weighed, and transferred to hermetically sealed containers of 1000mL, avoiding the passage of atmospheric air, but allowing respiratory flow to the samples. In each container was placed a smaller one with 10 mL of NaOH solution (1N) to capture CO2. At seven days intervals, the solutions were removed from the containers and were titrated. Four evaluations were realized. In the end, 2.5 mL BaCl (0.5 M) was added for precipitation and also 3 drops of the acid-base phenolphthalein indicator. The amount of CO2 released from the soil was considered after titration of excess NaOH with HCl solution (0.5 M). The calculation of respiration was done using the titration method with CO2 capture by NaOH by the formula:
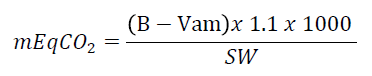
where:
B = volume of HCl used on titration of white treatment;
Vam = volume of HCl used on titration of the samples;
1.1 = conversion factor;
SW = soil weight.
The experiment was composed by 4 treatments: conventional, organic, conventional autoclaved and organic autoclaved, with 6 replicates each.
Statistical analysis
The microbial activity data was submitted to analysis of variance (ANOVA) (p≤0.05) using the Sisvar software (Ferreira, 2014). The distribution of the microbial population as a function of the microbial activity was subjected to the multivariate analysis by means of the analysis of covariance (ANCOVA) (p≤0.01) using the software Xlstat®.
RESULTS AND DISCUSSION
The analysis of variance results showed that CO2 soil evolution in two cultivation systems, expressed in mEqCO2.g-1, had a decrease in both cultivation systems studied, whether or not they have undergone the autoclaving process. However, at the beginning of the evaluations, it was detected that in the organic cultivation system there was higher microbial respiratory activity (Figure 1).
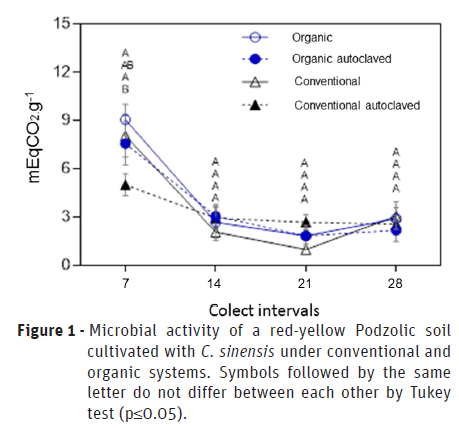
However, the soil microbial activity is a characteristic that has a close relation with the fertility and its management. In this way, it is important to observe the set of factors that converge for this. Thus, soil analyzes (Table 1) show that there are differences in fertility, which are a function of the adopted cultivation system.
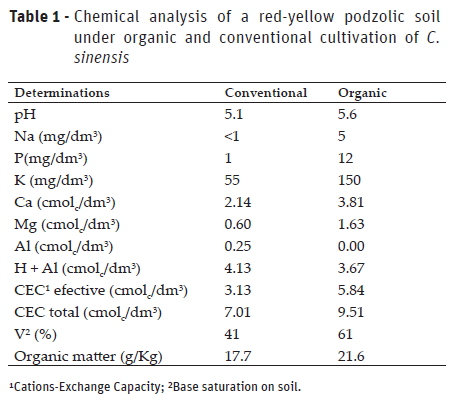
CTC rates are mediated by soil organic matter, as they are charged and consequently increased by CTC through nutrient retention, contributing to plant nutrition. Therefore, Montezano et al. (2004) refer that management of soil organic matter is critical to improve crop productivity. Thus, it is probable that the organic cultivation system presented greater microbial activity due to the greater maintenance of organic matter, a management that is not adopted in the area where the cultivation is done by conventional methods.
It is important to take attention to the soil management system, regardless of the cultivation system adopted, ho allows better productivity maintenance and soil quality (Carneiro et al., 2016). Another important aspect is the bases saturation, which is responsible for the increase of the soil pH. In this study it was verified that the organic cultivation system had better balance in its fertility when comparing to the conventional system.
Microbiological analyzes revealed differences between the microbial communities evaluated as actinomycetes and filamentous fungi. However, regardless the crop system, the soil bacterial population remained constant for all evaluated treatments, even with the autoclaving process, >2x108 CFU g-1 of soil (Table 2). Thus, the bacterial community in red-yellow podzolic soil can be considered a constant population not being affected by the cultivation system.
On the other hand, a differentiated behavior was observed between filamentous fungi and actinomycetes showing a population of 33.33x104 CFU g-1 in conventional cultivation and 181.66x104 CFU g-1 of soil in organic cultivation, respectively for this second group. Inversely, filamentous fungi population was more representative in conventional cultivation system, showing values of 21.83x104 CFU g-1 than in organic with values of 3.5x104 CFU g‑1 of soil. When the soil was autoclaved, the microbial population for these microorganisms was reduced. However, for actinomycetes the microbial population still remained considerably higher with a population of 96.83x106 CFU g-1 in organic soil, while the fungal population was drastically reduced independently of the cultivation system. These results show that actinomycetes have higher resistance to high temperatures and pressure (Figure 2). Thus, filamentous fungi and actinomycetes are groups of microorganisms that have important expression to determine the biological quality of soils as a function of the cultivation system.
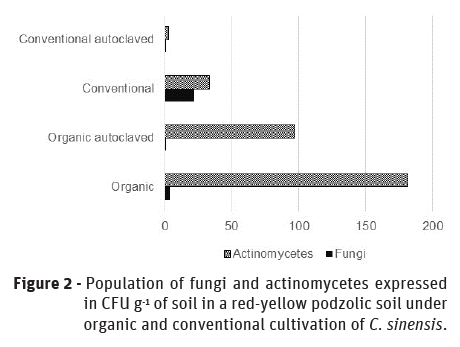
When studying soil microbial population, it is important to look at the factors together and not individually. In this way, it is known that the cultivation in organic system has its peculiarities, what differentiates it from the conventional one and makes it better from the ecological point of view. In the conventional cultivation system, for example, the application of agrochemicals causes imbalance in the microbial community.
So, it is essential the correct agricultural management regardless the system adopted and the crop of interest. Numerous factors contribute to the maintenance of soil fertility, like the population and microbial diversity to be observed, since several species of soil bacteria have the ability to fix atmospheric N and make it available to plants. Fungi, in turn, have the ability to survive in symbiosis with the roots and provide water and nutrients. Both these groups contribute significantly to plant nutrition, acting in addition to mineral fertilization. However, the maintenance of organic matter is of paramount importance, not only for the maintenance of the soil microbiota, but also for the improvement of chemical and physical attributes. In this aspect Spohn (2015) stands out the importance of maintaining organic matter, which is likely to improve the C:N ratio.
Crop rotations, especially those that include cover crops, sustain soil quality and productivity, increasing C, N and microbial biomass, making them a cornerstone for sustainable agroecosystems (McDaniel et al., 2014). On the other hand, in fruit crops of arboreal and perennial species it is difficult to include crop rotations, being necessary the adoption of alternatives that will collaborate with the improvement of the soil quality. Thus, cultivation of legumes can be recommended with subsequent incorporation into the soil.
CONCLUSIONS
Microbial diversity in soils cultivated with C. sinensis showed a high population of bacteria and actinomycetes. In organic cultivation, it is possible to find higher values related to microbial populations, which suffer less impact when submitted to high temperatures. In addition, the microbial community associated with microbial respiratory activity can be adopted as a strategy for the evaluation of biological quality in conventional and organic systems.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests
REFERENCES
Anderson, T.H. & Domsch, Z.H. (1993) - The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biology and Biochemistry, vol. 25, n. 3, p. 393-395. https://doi.org/10.1016/0038-0717(93)90140-7 [ Links ]
Balzergue, C.; Chabaud, D.; Barker, D.G.; Bécard, G. & Rochange, S.F. (2013) - High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Frontiers in Plant Science, vol. 4, art. 426. https://doi.org/10.3389/fpls.2013.00426 [ Links ]
Barroso, G.S.P.; dos Santos, T.M.C.; Montaldo, Y.C.; da Silva, J.M. & da Silva, P.J. (2012) - Respiração microbiana do solo cultivado com milho sobre dois sistemas de adubação no município de Rio Largo, Alagoas. Revista Verde de Agroecologia e Desenvolvimento Sustentável, vol. 7, n. 2, p. 8-10. [ Links ]
Campos, A.A.B.; Scotton, J.C.; Costa, W.L.F.; Giassi, V.; Pinto, D.F.P. & Homma, F.K. (2015) - Seleção de fungicidas visando a preservação de fungos micorrízicos arbusculares nativos no cultivo de feijoeiro. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 19, n. 9, p. 3936-3942. http://dx.doi.org/10.1590/1807-1929/agriambi.v19n9p898-902 [ Links ]
Carneiro, J.S.S.; dos Santos, A.C.M.; Fidelis, R.R.; da Silva Neto, S.P.; dos Ramos, A.C. & da Silva, R.R. (2016) - Diagnóstico e manejo da variabilidade espacial da fertilidade do solo no cerrado do Piauí. Revista de Ciências Agroambientais, vol. 14, n. 2, p. 10-21. [ Links ]
EMBRAPA (2014) - Sistema brasileiro de classificação de solos. 4ª ed. Brasília: DF, 376 p. [ Links ]
Fahy, P.C. & Hayward, A.C. (1983) - Media and methods for isolation and diagnostic test. In: Fahy, P.C. & Persley, G.J. (Eds.) - Plant bacterial diseases: a diagnostic guide. Sydney. Academic Press. pp. 337-338. [ Links ]
Ferreira, D.F. (2014) - Sisvar: a Guide for its Bootstrap procedures in multiple comparisons. Ciência e Agrotecnologia, vol. 38, n. 2, p. 09-112. http://dx.doi.org/10.1590/S1413-70542014000200001 [ Links ]
IBGE (2017) - Levantamento Sistemático da Produção Agrícola. Instituto Brasileiro de Geografia e Estatística. [cit. 2018.05.23] <https://sidra.ibge.gov.br/pesquisa/lspa/tablas> [ Links ]
Kuster, E. & Williams, S.T. (1964) - Selective media for the isolation of Streptomycetes. Nature, vol. 202, p. 928-929. [ Links ]
Martin, J.P. (1950) - Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Science, vol. 69, n. 3, p. 215-232. https://doi.org/10.1097/00010694-195003000-00006 [ Links ]
McDaniel, M.D.; Tiemann, L.K. & Grandy, A.S. (2014) - Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta‐analysis. Ecological Applications, vol. 24, n. 3, p. 560-570. https://doi.org/10.1890/13-0616.1 [ Links ]
Minatel, A.L.G.; Andrioli, I.; Centurion, J.F. & Willian, N. (2006) - Efeitos da subsolagem e da adubação verde nas propriedades físicas do solo em pomar de citros. Engenharia Agrícola, vol. 26, n. 1, p. 86-95. http://dx.doi.org/10.1590/S0100-69162006000100010 [ Links ]
Montaldo, J.C.; dos Santos, G.B.L.; Montaldo, Y.C.; dos Santos, T.M.C.; Barroso, G.S.P.; de Oliveira, J.U.L. & da Silva, J.M. (2018) - Basal respiration and microbial population of a red-yellow latosol under different harvesting systems of sugarcane. Global Science and Technology, vol. 11, n. 1, p. 8-16. [ Links ]
Montezano, Z.F.; Corazza, E.J. & Muraoka, T. (2006) - Variabilidade espacial da fertilidade do solo em área cultivada e manejada homogeneamente. Revista Brasileira de Ciência do Solo, vol. 30, n. 5, p. 839-847. http://dx.doi.org/10.1590/S0100-06832006000500010 [ Links ]
Sapp, M.; Harrison, M.; Hany, U.; Charlton, A. & Thwaites, R. (2015) - Comparing the effect of digestate and chemical fertilizer on soil bacteria. Applied Soil Ecology, vol. 86, p. 1-9. https://doi.org/10.1016/j.apsoil.2014.10.004 [ Links ]
Spohn, M. (2015) - Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences, vol. 12, p. 817-823. https://doi.org/10.5194/bg-12-817-2015 [ Links ]
Wang, C.; Wang, F.; Zhang, Q. & Liang, W. (2016) - Individual and combined effects of tebuconazole and carbendazim on soil microbial activity. European Journal of Soil Biology, vol. 72, p. 6-13. https://doi.org/10.1016/j.ejsobi.2015.12.005 [ Links ]
Wang, P.; Zhang, J.J.; Shu, B. & Xia, R.X. (2012) - Arbuscular mycorrhizal fungi associated with citrus orchards under different types of soil management, Southern China. Plant Soil and Environment, vol. 58, n. 7, p. 302-308. [ Links ]
Zhou, X.; He, Z.; Liang, Z.; Stoffella, P.J.; Fan, J.; Yang, Y. & Powel, C.A. (2011) - Long term use of cooper-containing fungicide affects microbial properties of citrus grove soils. Soil Science Society of America Journal, vol. 75, n. 3, p. 898-906. https://doi.org/10.2136/sssaj2010.0321 [ Links ]
ACKNOWLEDGMENTS
Federal University of Alagoas and Laboratory of Agricultural Microbiology and Soil.
Received/recebido: 2018.07.11
Accepted/aceite: 2018.12.10














