Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.42 no.2 Lisboa jun. 2019
https://doi.org/10.19084/rca.17177
ARTIGO
Sorption and desorption of chromium from applied tannery sludge in soils under pastures and Cerrado vegetation
Sorção e dessorção de cromo com o uso de lodo de curtume em solo com pastagem e vegetação de Cerrado
Rafael Felippe Ratke1, Mara L. Lemke-de-Castro², Alfredo B. De-Campos3, Cleonice Rocha4, Juliano M. Barbosa5, Adriana Verginassi6 and Alan M. Zuffo1,*
¹ Universidade Federal de Mato Grosso do Sul, Agronomic Engineering Departament, Chapadão do Sul, MS, 79650-000, Brazil
2 Universidade Estadual de Goiás, Department of Biological Sciences, Morrinhos, GO, 75650-000, Brazil
3 Universidade Estadual de Campinas, Institute of Geoscience, Campinas, SP, 13083-870, Brazil
4 Pontifícia Universidade Católica de Goiás, Department of Mathematics and Physics, Goiânia, GO, 74605-010, Brazil (In memoriam)
5 Instituto Federal de Roraima, Agronomic Departament, Caracaraí, RR, 69365-000, Brazil
6 Fazenda Nossa Senhora Aparecida, Agronomic Departament, Jataí, GO, 75804-092, Brazil
(* E-mail: alan_zuffo@hotmail.com)
ABSTRACT
Tannery sludge is often used in agriculture as a source of nutrients and for controlling soil acidity. However, the use of this substance can promote soil contamination by chromium. The objectives of this research were to determine the sorption and desorption isotherms of chromium from tannery sludge in a laboratory experiment. The sorption and desorption of chromium were determined in samples of an Oxisol under pasture and native vegetation (Cerrado). Soil samples were mixed with tannery sludge and a solution of chromium sulfate. The soil samples containing chromium at different concentrations were shaken for 72 hours in cycles of 12 hours of shaking and 12 hours of rest. The samples were then centrifuged, and the soil separated from the supernatant and were subjected to selective desorption with CaCl2, NaOH, and Na2EDTA solutions to determine the different fractions of chromium. Results showed of the chromium retention in the soil was strongly influenced by the soil organic matter and the organic matter from tannery sludge. That chromium presented in both tannery sludge and sulfate was strongly sorbed on soil. Chromium desorption was higher for samples treated with NaOH in the tannery sludge and with others extractors in the sulfate treatment.
Keywords: potentially hazardous elements, Freundlich, soil management.
RESUMO
O lodo de curtume é frequentemente usado na agricultura como fonte de nutrientes e para controlar a acidez do solo. No entanto, o uso dessa substância pode promover a contaminação do solo com cromo. O objetivo deste estudo foi determinar as isotermas de sorção e dessorção do cromo proveniente de lodo de curtume em um experimento de laboratório. A sorção e dessorção do cromo foram determinadas em amostras de um Latossolo sob pastagem e vegetação nativa de Cerrado. As amostras de solo foram misturadas com lodo de curtume e sulfato de cromo. As amostras de solo contendo cromo em diferentes concentrações foram agitadas por 72 h, alternando ciclos de 12 h de agitação e 12 h de repouso. As amostras foram centrifugadas, e o solo separado do sobrenadante foi submetido a extrações de dessorção seletiva com soluções de CaCl2, NaOH, e Na2EDTA para determinar diferentes frações de cromo. Os resultados mostraram que a retenção de cromo no solo foi fortemente influenciada pela matéria orgânica do solo. O cromo proveniente tanto do lodo de curtume como do sulfato foi fortemente sorvido no solo. A dessorção de cromo foi maior nas amostras tratadas com NaOH no lodo de curtume e com outros extratores no tratamento com sulfato.
Palavras-chave: materiais potencialmente perigosos, Freundlich, Manejo do solo
INTRODUCTION
The use of tannery sludge in agriculture can contribute to improve soil fertility and plant nutrition, the accumulation of organic matter in the soil (Konrad and Castilhos, 2002; Ferreira et al. 2003; Prado and Cunha, 2011), and help to this residue in the environment. However, the application of this residue in agriculture must be conducted with technical criteria, since its improper used can lead to high soil pH values and greater contents of soluble salts and chromium, which may compromise agricultural sustainability and the future use of these soils (Santos et al., 2014).
Tannery sludge is composed of organic materials from animals and inorganic salts; some of these components are nutrients (nitrogen, calcium, sulfur, phosphorus, magnesium and potassium) for plants and microorganisms (Santos et al., 2014).
Tannery residues, especially sludge from sewage treatment stations, are used in agriculture as a soil conditioner to reduce soil acidity thus; reducing the exchangeable aluminum content, increasing soil pH, and in some cases, it is a substitute to lime. The effect of these industrial residues as a soil conditioner is due to their significant contents of carbonates, especially calcium, and hydroxides, from the industrial liming and unhairing of the leather (Ferreira et al., 2003; Teixeira et al., 2006; Prado and Cunha, 2011).
Konrad and Castilhos (2002) has shown the application of 20.5 Mg ha-1 (dry basis) of tannery sludge raises the soil pH from 4.5 to 5.5 and increase in six times the calcium content an Ultisol.
Despite the beneficial effects of tannery sludge application on soil fertility (Ferreira et al. 2003), there is a potentially harmful effect on the soil and environment due to an increase in soil salinity (Konrad and Castilhos 2002) and chromium concentration from the sludge. Gupta et al. (2007) showed the use of fertigation with tannery sludge that has large concentrations of chromium and iron causes pollution of agricultural soils and groundwater. The potential of soil and water contamination by chromium depends on soil properties. Lemke-De-Castro et al. (2015) has shown the competitive sorption of cadmium and chromium depends on the soil class and mineralogy. Little is known about the behavior of Oxisols on sorption and desorption of chromium. Oxisols present mineralogy with large concentration of kaolinite and hematite (Gomes et al., 2004). Therefore, these soils have large amounts of iron oxides, whose surface charges vary with the pH, affecting the sorption and desorption of metals in the soil. Thus, hypothetically chromium may be retained in Oxisols.
The use of selective extraction methods may explain the behavior of chromium in Oxisols from Cerrado (Marques et al., 2004). Mandal et al. (2011) reported that 11% of the chromium (III) was in the mineral fraction of soils that received tannery sludge from fertigation, then Cr can be easily leached and absorbed by plants. Although a few studies have been conducted on the behavior of applied tannery sludge rich chromium in Oxisols, it is still not known the sorption and desorption rates of chromium on this soil type.
The hypothesis tested in this research were that, at low concentrations, the total Cr is absorbed and, at high concentrations, the sorption of Cr decreases; and that the complex matrix of tannery sludge related to total Cr promotes different sorption and desorption depending on the compound used.
In this context, the objective of this study was to determine the sorption and desorption isotherms of total Cr from tannery sludge, using a control solution of chromium sulfate, on an Oxisol under pasture and native vegetation (Cerrado). The application of tannery sludge to the soil as a means of discarding this industrial residue is frequent, thus, it is of interest to assess the chromium activity in the soil after these applications, in order to reduce their harmful effects.
MATERIAL AND METHODS
The experiment was conducted in the Laboratory of Soil and Leaf Analysis of the School of Agronomy and Food Engineering and in the Laboratory of Geology and Physical Geography of the Federal University of Goiás, Goiânia, State of Goiás (GO), Brazil. The experimental design used the completely randomized model, with three replications. Two sources of chromium (chromium sulphate solution only and tannery sludge) and two soils (under pasture with Tifton grass and native Cerrado vegetation) were evaluated. The soil was an Oxisol (classified according to Soil Survey Staff, 2014) with clay-loam texture and mineralogy dominated by kaolinite and hematite.
The soil samples were collected in a rural property (16º30‘30"S, 49º15‘59"W), at a depth of 0.20-0.40 m (sub-surface horizon) with a Dutch auger, at a single point for each type of vegetation cover. Soil samples were air-dried, crushed, and sieved through a 2 mm mesh sieve for chemical-physical analysis. The soil chemical parameters were determined: pH in CaCl2 (1:2.5) in each sample; the potential acidity (H+Al), extracted in calcium acetate buffered at pH 7.0 and quantified by titration with NaOH; P, K+, Cu2+, Fe2+, Mn2+, Zn+ e Cr total extracted with Mehlich-1; Ca2+ and Mg2+, extracted with KCl 1 mol L-1. The granulometric analysis (clay, silt and sand) was performed by means of mechanical dispersion and stabilization of the sample by means of a stirrer in a dispersant solution (NaOH 1mol L-1), followed by separation of the fractions by sieving and sedimentation. The organic matter (MO) was determined by wet oxidation with potassium dichromate in a sulfuric medium, followed by the titration with unheated ammoniacal ferrous sulfate, without correction factor. With these results, the following parameters were calculated cation exchange capacity (CEC = Ca + Mg + K + H + Al) and described in Table 1.
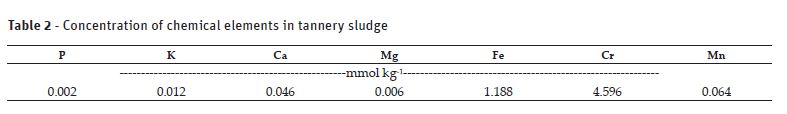
The sludge was collected in the drying bed of a tannery in Senador Canedo-GO, which processes bovine skin to the chromium tanning stage. The tannery sludge was homogenized and a sample of 2.5 ml was collected, this sample was chemically analyzed for the parameters: P, K+, Ca2+, Mg2, Fe2+, Mn2+ e total Cr. The sample of tannery sludge was subjected to acid digestion (perchloric nitrate, 1:6 ratio). The concentration of Ca2+, Mg2, Fe2+, Mn2+, Zn+ e Cr total in soil and in the sludge were determined by atomic absorption spectrophotometry. P and K were determined by colorimetry and flame photometry. The physicochemical analysis of the soil and the sludge were carried out following the methodology described by Silva (2011).
A basic di-hydrated chromium sulfate (Cr4(SO4)5(OH)2) with 25% of Cr2O3 was used to carry out the sorption and desorption experiment. The chromium sulfate solutions were diluted in deionized water to chromium concentrations of 0 (control), 0.15, 0.31, 0.63, 1.26, 1.89, 2.20 and 5.51 mmol L-1 then added to tannery sludge. Aliquots of 10 mL of chromium contaminated tannery sludge were added to centrifuge tubes containing soil to a ratio of 1:40 (soil/solution) (m/V). The solutions were added to 0.00025 kg of homogenized dry soil from both locations. The tubes remained at rest for one hour then were shaken with a glass rod and their pH was measured. The pH was adjusted to 5.5 by adding either HCl (10 mmol L-1) (pH>5.5) or NaOH (10 mmol L-1) (pH<5.5) solution. The solutions were then shaken for 72 hours on a mechanical shaker at 192 rpm, alternating cycles of 12 hours of shaking and 12 hours of rest. The suspension from each tube was centrifuged for 20 minutes at 3000 rpm and the supernatant removed for determination of chromium content.
The amount of chromium in the supernatant of the solutions determined by atomic absorption spectrophotometry with flame atomization (PerkinElmer AAnalyst 100), using compressed air and acetylene gas. The total chromium of the solution was determined using the wavelength of 186 nm. After the sorption procedures, the chromium sorbed in the soil was calculated according to the Equation 1

in which q is the amount of chromium sorbed in the soil (mmol kg-1), v is the volume of added solution (mL), Ci is the initial chromuium concentration of the solution (mmol L-1), Ceq is the concentration of chromium in equilibrium in the sorption test (mmol L-1), and ms is the weight of the soil (kg). The total sorption of chromium for each concentration and soil sample was calculated by the difference between the amounts of chromium added to that present in the supernatant.
After the sorption experiment test, the samples were subjected to selective desorption procedures adapted from Rao et al. (2008) to determine the removal of the chromium bonded to the soil during the sorption test. The first procedure aimed at determining the desorption of water-soluble and capable of ionic exchanges chromium (exchangeable fraction). To this end, 25 mL of a CaCl2 solution (15 mmol L-1) was added to the centrifuge tubes containing the remaining soil from the sorption test. After resting for one hour, the contents of these tubes were shaken with a glass rod and the pH of the suspension was measured and adjusted to 5.5, as described in the sorption experiment.
After adjusting the pH, the tubes were shaken for 72 hours on a mechanical shaker at 192 rpm, alternating cycles of 12 hours of shaking and 12 hours of rest. After 72 hours of rest, the suspension of each tube centrifuged at 3000 rpm for 20 minutes and the supernatant removed for determination of chromium content.
After desorption of the water-soluble and exchangeable chromium fractions of these samples, desorption of chromium bonded to soil organic matter was determined. To perform this, 2.5 mL of a NaOH solution (0.5 mol L-1) was added to each centrifuge tube containing the remaining soil from the previous step then shaken with the glass rod and after by a mechanical shaker at 192 rpm for. The tubes containing the NaOH solution shaken for 16 hours by a mechanical shaker at 192 rpm. The suspension of each tube was then centrifuged at 3600 rpm for 45 minutes and the supernatant removed for chromium determination.
The desorption of chromium bonded to soil inorganic materials, mainly mineral phases, was determined by adding 2.5 mL of a Na2EDTA solution (0.05 mol L-1) to each centrifuge tube containing the soil remaining from the previous steps. The tubes were shaken for one hour on a mechanical shaker at 192 rpm, and the suspension of each tube was centrifuged at 3600 rpm for 45 minutes and the supernatant removed for chromium determination. The total desorption of chromium was calculated by the difference between the amount of chromium in the supernatant and the amount of chromium sorbed to the soil. The desorbed chromium was determined by using the same equipment and conditions described in the sorption tests.

in which qe is the amount of chromium adsorbed at equilibrium (mmol kg-1), Ce is the chromium concentration at equilibrium (mmol L-1), KF is the constant of chromium sorption capacity (mmol kg-1) and n is the constant of chromium sorption intensity (considered favorable at the range of 2 to 10).
The linear equation form of the Freundlich equation described in the Equation 3,
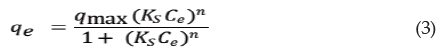
The angular and linear coefficients of the lines were used to determine the values of the Freundlich parameters (KF and n). The sorption capacity of chromium in the solid phase of the soil was graphically described by using the partition coefficient (Kd) [17], as described in the Equation 4,

in which q is the amount of chromium absorbed and Ce is the coefficient of equilibrium of the solution.
Linear regressions were fitted to the chromium contents in the contaminant solution (independent variable) and its concentration in the filtered solution after equilibrium (dependent variable). The F was test used to evaluate the significance of the regression parameters.
RESULTS AND DISCUSSION
The sorption isotherms of chromium generated by the Freundlich equation showed type C (Figure 1).
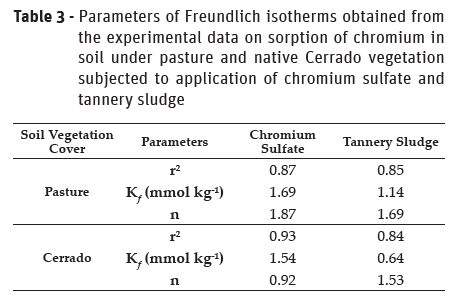
The Freundlich equations presented significant linear polynomial regressions for the chromium concentrations in different solutions and soils (Table 3). The values of n in the Freundlich equation were below 2, indicating a low intensity of sorption of chromium in the soil under pasture and Cerrado when applying chromium sulphate and tannery sludge as proposed by Shinzato et al. (2009).
Stadella et al. (2008) showed that sorption of chromium increases with increasing pH. Alcântara and Camargo (2001) reports lated that the liming raises the pH of an Oxisol then favoring sorption of Cr (III). In the present study, the adjustment of the pH to 5.5 promoted conditions of maximum sorption of chromium in the soil.
The partition coefficients (Kd) for sorption of chromium in the soil showed that the higher the chromium concentration in the equilibrium solution the lower the Kd value in both soils (Figure 2). However, the Kd of the soil under pasture was about twice as high with the application of chromium sulfate. These results support that idea that the chromium applied to the soil as chromium sulfate is differently sorbed from the chromium of tannery sludge due to the intrinsic biogeochemical characteristics of the tannery residue and the soils under of pasture. For instance, the soil under pastures received limestone and fertilizers to make the soil condition the soil the better in terms of availability and replacement of nutrients thus maintaining the productive potential of the feed for animals. However, these additions influence the chemical balance of the soil and the sorption of elements such as Cr.
It is reported that the mobility of metals from the soil may be lower due to the presence of organic matter in the soil (Hernandez-Soriano et al., 2008). Thus, metals are often bonded to organic matter and retained in the soil. The higher Kd of the sorption of chromium when using tanning sludge is probably related to the complexation of this metal to the soil organic matter.
The organic matter content related to the light fraction of carbon is higher in Oxisol under native Cerrado vegetation than under pasture areas (Carneiro et al., 2009). In the present study, the Kd of the soil under native Cerrado vegetation (MO = 34.0 g kg-1) increased with high chromium concentration in the solution. Allison and Allison (2005) found an average Kd values of 3.9 in soils and 5.1 in sediments for chromium, thus showing that this element has higher sorption energy when combined with organic matter. Therefore, the organic matter presented in the soil under native Cerrado vegetation affected the Kd results in our study, favoring the sorption of this metal.
The chromium desorption isotherms from both soils were type C, even when using different extractors (Figure 3), which confirms Essington (2004). However, the amplitude and significance of the linear regression of these isotherms were different between the soils evaluated, i.e. applied chromium solutions and extracted aqueous substances used in this experiment (Table 4). Covelo et al. (2007) showed that the Freundlich equation is efficient to describe desorption of chromium in the soil. Thus, the desorption isotherms experiment from our study is a reliable indicator of what happens with chromium in the soil.
The extraction of chromium from the soil under native Cerrado vegetation and pasture with CaCl2 solution showed linear regressions with low concentrations of chromium in the equilibrium solution (Figure 3, A and B). The CaCl2 solution was not efficient for extracting all sorbed chromium. This may have occurred because chromium forms complex with minerals from the soil, in agreement with Lemke-De-Castro et al. (2015) who reports that chromium form inner sphere complexes with iron oxides, which are the sorption sites for metals in Oxisols.
Desorption of chromium in NaOH solution increased with increasing chromium concentration in the equilibrium solution when the tanning sludge was applied in both soils (Figure 3, C and D). However, when chromium sulfate was applied to the soil, the chromium desorption occurred with low chromium concentrations in the equilibrium solution. Therefore, chromium from chromium sulfate was not sorbed to sorption sites of the soil organic matter. However, all treatments presented isotherms with significant linear regressions which showing that the increasing of chromium in equilibrium increased the desorption of chromium in the soil under pasture and native Cerrado vegetation, regardless the source of chromium used (chromium sulfate and tannery sludge).
In order for NaOH to extract chromium, its concentrations must be high. This fact indicates that chromium bounded to organic matter is not solubilized in water or extracted in CaCl2. However, not all organic matter is soluble; the fractionation of organic matter with NaOH divides it into three compounds, humic acids, fulvic acids and humina, the latter being insoluble (Dick et al., 2009). Thus, in this study chromium probably remained in the humina fraction of organic matter. The organic matter may have formed inner sphere complex with chromium, as described by Essington (2004).
Chromium from applied tannery sludge remained complexed to the organic matter in the soil, which affected the desorption of this metal (Rafatulliah et al., 2009). Chromium can also be complexed to the tanning mud itself. Chromium bonds to low-solubility compounds related to cutaneous tissue proteins that is released in the mud, whose release is slow (Aquino-Neto and Camargo, 2000).
The extraction of chromium by Na2EDTA showed the sorption of this metal in the mineral fraction of the Oxisol. The desorption isotherm of chromium was dependent on the sources of chromium applied and the soil used (Figure 3 E and F). According to the isotherms results, the lower the concentration of chromium in the solution of equilibrium the greater is its desorption when using chromium sulfate in the soil under native Cerrado vegetation. On the other hand, the tannery sludge requires a higher concentration of chromium in the equilibrium solution for chromium to be desorbed, and the increase of chromium in the equilibrium solution does not favor the increase of desorption of chromium in the soil under pasture.
The total desorption of chromium was higher in the soil under pasture than in the soil under native Cerrado vegetation. Therefore, the total desorption of chromium from chromium sulfate and tannery sludge was different for the two soils (Figure 3 G and H, Table 4). Chromium in the soil under pasture showed greater desorption when tannery sludge was used. The increase in the concentration of chromium at the equilibrium solution caused an hight in desorption for both solutes used thus presenting significant linear regressions. However, desorption of chromium was not significantly different with the use of tannery sludge and chromium sulfate in the soil under native Cerrado vegetation.
According to McBride (1994), chromium can be retained in the soil with pH < 7 by inner-sphere complex, as found for the soils of the present study. Lemke-De-Castro et al. (2015) reports that the sorption and desorption of chromium are dependent on the mineral composition of the soil, which is favorable in soils with the presence of iron oxides, such as the Oxisol used in the present study. Covelo et al. (2007) showed that the retention of chromium in the soil is also related to the type of organic matter and clay that are present in the soil and that chromium is retained in humified organic matter, kaolinite and vermiculite. Thus, kaolinite in the studied soil was probably a sorption site for chromium together with the organic matter and iron oxides.
CONCLUSIONS
Soil organic matter is the main site to promote retention of chromium in Oxisol.
The sorption isotherms of chromium in Oxisol under pasture and native Cerrado vegetation is dependent on the source of chromium.
Freundlich desorption isotherms for chromium in Oxisol are of type C shape, with different amplitudes depending on the soil management and the use of tannery sludge.
The use of chromium sulfate to evaluate the sorption and desorption of this metal in the soil is different of the use of residues containing high amounts of organic matter, such as tannery sludge.
References
Aquino Neto, V. & Camargo, O.A. (2000) - Crescimento e acúmulo de crômio em alface cultivada em dois latossolos tratados com CrCl3 e resíduos de curtume. Revista Brasileira de Ciência do Solo, vol. 24, n. 1, p. 225-235. https://doi.org/10.1590/s0100-06832000000100025 [ Links ]
Alcântara, M.A.K. & Camargo, F.O.A. (2001) - Isotermas de adsorção de Freundlich para o crômio (III) em Latossolos. Scientia Agricola, vol. 58, n. 3, p. 567–572. https://doi.org/10.1590/s0103-90162001000300020 [ Links ]
Allison, J.D. & Allison, T.L. (2005) - Partition Coefficients for Metals in Surface Water, Soil, and Waste. Washington: US Environmental Protection Agency. [ Links ]
Carneiro, M.A.C.; Souza, E.D.; Reis, E.F.; Pereira H.S. & Azevedo, W.R. (2009) - Atributos físicos, químicos e biológicos de solo de Cerrado sob diferentes sistemas de uso e manejo. Revista Brasileira de Ciência do Solo, vol. 33, n. 1, p. 147–157. http://dx.doi.org/10.1590/S0100-06832009000100016 [ Links ]
Covelo, E.F.; Vega, F.A. & Andrade, M.L. (2007) - Simultaneous sorption and desorption of Cd, Cr, Cu, Ni, Pb, and Zn in Acid Soils. Journal of Hazardous Materials, vol. 147, n. 3, p. 862–870. https://doi.org/10.1016/j.jhazmat.2007.01.123 [ Links ]
Dick, D.P.; Novotny E.H.; Diecknow J. & Bayer, C. (2009) - Química Da Matéria Orgânica do Solo. In: Melo, V.F. & Alleoni, L.R.F. (Eds) - Química E Mineralogia Do Solo: Parte II – Aplicações. Viçosa: Sociedade Brasileira de Ciência do Solo, p. 1–69. [ Links ]
Essington, M.E. (2004) - Soil and Water Chemistry: An Integrative Approach. Boca Raton: Taylor & Francis publishing group. [ Links ]
Ferreira, A.S.; Camargo, F.A.O.; Tedesco, M.J. & Bissani, C.A. (2003) - Alterações de atributos químicos e biológicos de solo e rendimento de milho e soja pela utilização de resíduos de curtume e carbonífero. Revista Brasileira de Ciência do Solo, vol. 27, n. 4, p. 755–763. https://doi.org/10.1590/S0100-06832003000400020 [ Links ]
Gomes, J.B.V.; Curi, N.; Schulze, D.G.; Marques, J.J.G.S.M; Ker, J.C. & Motta, P.E.F. (2004) - Mineralogia, morfologia e análise microscópica de solos do bioma Cerrado. Revista Brasileira de Ciência do Solo, vol. 28, n. 4, p. 679–694. https://doi.org/10.1590/s0100-06832004000400010 [ Links ]
Gupta, A.K.; Sinha, S.; Basant A. & Singh, K. P. (2007) - Multivariate Analysis of Selected Metals in Agricultural Soil Receiving UASB Treated Tannery Effluent at Jajmau, Kanpur (India). Bulletin of Environmental Contamination and Toxicology, vol. 79, n. 5, p. 577–582. https://doi.org/10.1007/s00128-007-9287-3 [ Links ]
Hernandez-Soriano, M.C.; Degryse, F. & Smolders, E. (2008) - Heavy Metal Availability in Soil in the Presence of Anionic Surfactants. Communications in Agricultural and Applied Biological Sciences, vol. 73, n. 1, p. 157–161. [ Links ]
Konrad, E.E. & Castilhos, D.D. (2002) - Alterações químicas de solo e crescimento de milho decorrente adição de lodo de curtume. Revista Brasileira de Ciência do Solo, vol. 26, n. 1, p. 257–265. https://doi.org/10.1590/s0100-06832002000100027 [ Links ]
Lemke-De-Castro, M.L.; Borges J.D. & Leandro W.M. (2015) - Sorção competitiva entre cádmio e cromo em Latossolo variando pH e Eletrólito de suporte. Revista Brasileira de Ciências Agrárias, vol. 10, n. 3, p. 396–402. https://doi.org/10.5039/agraria.v10i3a5019 [ Links ]
Mandal, B.K.; Vankayala R. & Kumar L.U. (2011) - Speciation of Chromium in Soil and Sludge in the Surrounding Tannery Region, Ranipet, Tamil Nadu. Toxicology, vol. 2011, art.. 697980. https://doi.org/10.5402/2011/697980 [ Links ]
Marques, J.J.; Schulze D.G.; Curi N. & Mertzman, S.A. (2004) - Trace Element Geochemistry in Brazilian Cerrado Soils. Geoderma, vol. 121, n. 1–2, p. 31–43. https://doi.org/10.1016/j.geoderma.2003.10.003 [ Links ]
Mcbride, M. (1994) - Trace and Toxic Elements in Soils. In: Mcbride, M. (Ed). - Environmental Chemistry of Soil. New York: Oxford University Press. [ Links ]
Prado, A.K. & Cunha, M.E.T. (2011) - Efeito da aplicação de lodo de esgoto e curtume nas características físico-químicas do solo e na absorção de nitrogênio por feijoeiro. UNOPAR Científica Exatas Tecnologica, vol. 10, n. 1, p. 37–41. [ Links ]
Rafatullah, M. & Sulaiman, O.; Hashim R. & Ahmad, A. (2009) - Adsorption of Copper (II), Chromium (III), Nickel (II) and Lead (II) Ions from Aqueous Solutions by Meranti Sawdust. Journal of Hazardous Materials, vol. 170, n. 2–3, p. 969–977. https://doi.org/10.1016/j.jhazmat.2009.05.066 [ Links ]
Rao, C.R.M.; Sahuquillo A. & Sanchez. J.F.L. (2008) - A Review of the Different Methods Applied in Environmental Geochemistry For Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water, Air, and Soil Pollution, vol. 189, n. 1–4, p. 291–333. https://doi.org/10.1007/s11270-007-9564-0 [ Links ]
Santos, J.L.; Araújo A.S.F.; Nunes, L.A.P.L.; Oliveira M.L.J. & Melo, W.J. (2014) - Chromium, Cadmium, Nickel, and Lead in a Tropical Soil after 3 Years of Consecutive Applications of Composted Tannery Sludge. Communications in Soil Science and Plant Analysis, vol. 45, n. 12, p. 1658–1666. https://doi.org/10.1080/00103624.2014.907913 [ Links ]
Shinzato, M.C.; Montanheiro, T.J.V.A.; Janasi, S.A. & Yamamoto, J.K. (2009) - Remoção de Pb2+ e Cr3+ em solução por zeólitas naturais associadas a rochas eruptivas da formação serra geral, bacia sedimentar do Paraná. Química Nova, vol. 32, n. 8, p. 1989–1994. https://doi.org/10.1590/S0100-40422009000800002 [ Links ]
Silva, F.S. (2011) - Manual de análises químicas de solos, plantas e fertilizantes. Embrapa, 2a edição. Brasília, DF. 627 p. [ Links ]
Soil Survey Staff (2014) - Keys to Soil Taxonomy. 12th ed. USDA-Natural Resources Conservation Service, Washington, DC. [ Links ]
Stadella, C.C.; Pocrifka J.R.O. & Cossich, E.S. (2008) - Efeito da utilização de solução tampão sobre a biossorção de cromo (III) pela biomassa da alga marinha Sargassum sp. Acta Scientiarum. Technology, vol. 25, n. 1, p. 77–82. https://doi.org/10.4025/actascitechnol.v25i1.2251 [ Links ]
Teixeira, K.R.G.; Gonçalves Filho, E.M.S.; Carvalho, A.S.F.; Araújo, L. & Santos, V.B. (2006) - Efeito da adição de lodo de curtume na fertilidade do solo, nodulação e rendimento de matéria seca do caupi. Ciência e Agrotecnológia, vol. 30, n. 6, p. 1071–1076. https://doi.org/10.1590/s1413-70542006000600004 [ Links ]
Acknowledgments
The authors thank the professor Dr. Wilson Mozena Leandro of the Universidade Federal de Goiás for his collaboration in the laboratory analysis of the materials used in this study.
Received/recebido: 2018.09.25
Accepted/aceite: 2019.01.02














