Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciências Agrárias
versão impressa ISSN 0871-018X
Rev. de Ciências Agrárias vol.42 no.3 Lisboa set. 2019
https://doi.org/10.19084/rca.17279
ARTIGO
Physicochemical and sensorial characterization of commercial sugarcane syrups
Caracterização físico-química e sensorial de melados comerciais de cana-de- açúcar
Carolina M. Vicentini-Polette1,*, Jhéssica S.A.H.S. Belé1, Maria Teresa M.R. Borges1, Marta H.F. Spoto2 and Marta R. Verruma-Bernardi1
1 Programa de Pós Graduação em Agroecologia e Desenvolvimento Rural, Universidade Federal de São Carlos, Centro de Ciências Agrárias. Rodovia Anhanguera km 174, Araras, SP, Brasil
2 Universidade de São Paulo, Escola Superior de Agricultura Luiz Queiroz. Avenida Pádua Dias, 11, Piracicaba, SP, Brasil
(*E-mail: camvicentini@usp.br)
ABSTRACT
The aim of this study was to obtain information about the physicochemical and sensorial characteristics of sugarcane syrup. Fifteen brands were evaluated for pH, acidity, soluble solids, viscosity, moisture, total solids, reducing sugars, ash and instrumental color. Five brands were evaluated for sensory characteristics, preference and purchase intention. The physicochemical and sensorial data were evaluated by analysis of variance (ANOVA) and Tukey's test (p≤0.05) and Friedman test (p≤0.05), respectively. Results showed the following average values: 4.7 for pH; 0.70% total acidity; 78.7 ° Brix for soluble solids; 116 St of viscosity; 18.1% moisture content; 81.9% total solids; 38.0% reducing sugars; 1.6% ash; and L* a* b* colors, respectively, 21.0, 1.2 and 2.4. The sensorial viscosity corroborated the instrumental viscosity and the soluble solids content. There was no differentiation for the sweet taste. The samples with the highest purchase intention and preference were the darkest sensorially, very viscous in appearance and with low or moderate texture viscosity. There is a wide variation between the physicochemical parameters of sugarcane syrup, not necessarily implying a technological quality problem, and it is suggested the revision of the range values stipulated in the legislation.
Keywords: food and nutrition, natural food, food composition, sugarcane.
RESUMO
O objetivo do estudo foi obter informações sobre as características físico-químicas e sensoriais de melados de marcas comerciais. Foram avaliados 15 melados quanto ao pH, acidez, sólidos solúveis, viscosidade, humidade, sólidos totais, açúcares redutores, cinza e cor instrumental. Cinco marcas foram avaliadas quanto às características sensoriais, preferência e intenção de compra. Os dados físico-químicos obtidos foram avaliados por análise de variância e teste de Tukey (p≤0,05), e os sensoriais por teste de Friedman (p≤0,05). As médias foram: 4,7 para pH; 0,70% de acidez total; 78,7°Brix para sólidos solúveis; 116 St de viscosidade; 18,1% de umidade ; 81,9% de sólidos totais; 38,0% de açúcares redutores; 1,6% de cinza; cor L, a*, b*, respectivamente, 21,0, 1,2 e 2,4. Houve relação da viscosidade sensorial com a instrumental e o teor de sólidos solúveis. Não houve diferenciação para gosto doce. As amostras com maior intenção de compra e preferência foram as mais escuras sensorialmente, aparência bastante viscosa e textura de pouca ou média viscosidade. Há uma grande variação entre os parâmetros físico-químicos de melados de cana-de-açúcar, não significando necessariamente um problema de qualidade tecnológica, e sugere-se revisão das faixas de valores estipuladas na legislação.
Palavras-chave: alimentos e nutrição, alimentos naturais, composição de alimentos, cana-de-açúcar.
INTRODUCTION
The sugarcane syrup is defined as the product obtained by the concentration of the sugarcane juice (Saccharum officinarum L.) or from the melted “rapadura”, the sugarcane candy (Brasil, 2005). For the production of sugarcane syrup, the cane juice, or “garapa”, is concentrated from 2.5 to 4 times, depending on the amount of sugar initially present in the juice.
The juice quality is directly related to the sucrose concentration and the purity of the raw material, the sugarcane juice, extracted from the culms by grinding, and being an impure and diluted sucrose solution, contains about 75-82% of water and 18-25% of soluble solids (Jeronimo, 2018). The soluble solids are grouped into sugars - sucrose, glucose and fructose, with the respective ratios: 14.5-23.5, 0.2-1.0 and 0.0-0.5%, in addition to the organic (0, 8-1.5%) and inorganic (0.2-0.7%) no-sugars (Stupiello, 1987cited by Jeronimo, 2018).
The sensory characteristics of sugarcane syrup are the syrupy and dense (viscous) liquid appearance, colored amber-yellow, with its own scent and sweet taste, and is different from molasses, the latter being described as the liquid obtained as a byproduct of crystallized sugar manufacturing (Brasil, 2005).
Recently the search for unrefined and natural products has had a great increase in the world's more developed areas. This is related to the fear many consumers have over artificial sweeteners, which are considerably sweeter than sucrose and fructose - although these sweeteners are a good choice for diabetes control, many believe they are unhealthy (Eggleston, 2018). Consumer's attitude towards labeling and certification of organic products has a positive impact on consumer confidence over the food and on the purchase intention of it (Liang, 2016). As discussed by De Sousa et al. (2012) some studies have shown that organic food may be highlighted by lower toxicity, longer shelf life and nutrient content in specific cases, but more comparative studies must be performed to prove the superiority of its nutritional value.
Moratoya et al. (2013) claimed that the three most consumed foods in the world are cereals, vegetables and milk, while in Brazil it is possible that the most consumed items are cereals, milk and fruit, in that order. With the evolution of medical science and the consequent search for higher quality lifestyles, new habits associated to a healthier diet have been sought by a growing people contingent from developed countries with reflexes in all corners of the world (Madail et al., 2011).
The sugarcane syrup is highly demanded in the natural food market, being an energetic and nutritious food, and preserving the nutrients from the cane juice, such as minerals: iron, calcium, potassium, sodium, phosphorus, magnesium and chlorine (Nogueira et al., 2009). Since production of sugarcane syrup does not involve centrifugation, unlike what happens in the sugar - as many of the micronutrients are removed in this process, the nutritional value of thissyrup is considerably high, and therefore it is shown as an important food for society (Eggleston, 2018).
The cane syrup can be consumed pure or added to other foods, and it has great nutritional importance in several Brazilian regions, with each 100g of the product providing about 300 calories and an important amount of minerals and vitamins (Jeronimo, 2018).
Information about food composition is necessary for the evaluation of the diet quality, besides allowing and improving the development and application of dietary guidelines in the public health nutrition field (Elmadfa e Meyer, 2010).
According to the UNICAMP (2011) Food Composition Table in 100g of sugarcane syrup there are 309 kcal, 22.1% moisture, 76.6 g carbohydrate, 1.3 g ash and no measures of dietary fiber and cholesterol, and does not contain protein, lipids and fibers. As for the minerals, it has calcium (102mg), magnesium (115mg), iron (5.4mg), manganese (2.62mg), phosphorus (74mg) and mostly potassium (395mg) (Unicamp, 2011).
Although not popularly known for its calcium content, sugarcane syrup presents in 5 tablespoons (20g) the equivalent of 100mL of whole cow's milk, a food widely known by the calcium content. As for the magnesium content, the sugarcane syrup approximates to the found in spinach for equivalent amounts - in each 100g, the sugarcane syrup supplies 115mg of the mineral, while the braised spinach provides 123mg (Unicamp, 2011). Thus, just one spoon of sugarcane syrup (20g) contains 61.8kcal, 15.3g of carbohydrates, 20.4g of calcium and 23g of magnesium, being a product with nutritional significance.
Besides the nutritional value, the sugarcane syrup has economic importance, mainly for family groups. Representing the production of sugarcane syrup in Brazil, Sulzbacher & David (2009) showed the reality of a family agribusiness where members would traditionally produce food derived from sugarcane, mainly the cane syrup, "schimier" and cane candy for the subsistence of the family group. However, from the perception of the existence of a growing consumer market that was willing to pay for a cultural, quality and differentiated product, the interest of the family group was aroused in expanding the activity and transforming its artisanal production into a sugarcane processing agroindustry. This change was significant for the family, increasing their income and helping them to improve the life quality.
This study aimed to establish a standard of technological quality and identity of sugarcane syrup, as well as specific technological aspects and nutritional information, in order to provide relevant information to consumers, health professionals and industry, enabling a better use and appreciation of product.
MATERIAL AND METHODS
Fifteen brands of sugarcane syrup were purchased containing in their label the designation (product name), company name and address of the manufacturer, net weight, ingredients and expiration date. Those with validity higher than the duration of the experiment (8 months) were selected, although the interval between the manufacturing and the validity was not ºdetermined given the lack of information in some samples.
Physicochemical analysis
Physicochemical analyzes of pH, acidity, soluble solids, viscosity, moisture, total solids, reducing sugar and ash were performed.
The pH determination was performed in 10% solution at 25°C, in a digital pHmeter model Tecnal TEC-2MP, and the acidity was determined by the sample titration in 10% solution with 0.01 M standardized sodium hydroxide (NaOH) solution until observation of the visual endpoint (ICUMSA, 2011). The results were presented in %, with aconitic acid as equivalent. Both analyzes were performed in triplicate.
The determination of the soluble solids content (° Brix) was performed in a Reichert r2300 automatic refractometer at 20°C, in triplicate (ICUMSA, 2011).
The viscosity was determined in Ford Cup, orifice 08, at 23°C, in triplicate. The beaker was filled with the sample, leveled, and the flow time (seconds) orifice opening to the first interruption or sudden thinning of the flow was recorded, according to NBR 16477 (ABNT, 2016). The results were transformed into kinematic viscosity values (St).
The evaluation of total solids and moisture was performed gravimetrically by direct drying in an oven at 100-105ºC (IAL, 2008), being the syrup deposited on calcined sand, in duplicate.
The concentration of reducing sugars (RS) was determined according to Lane-Eynon's methodology (IAL, 2008), using as a standard a 5% glucose solution, in duplicate.
The ash content was obtained by gravimetry, through sample dehydration and subsequent furnace burning, in duplicate (IAL, 2008).
The instrumental color of the cane syrup was evaluated in duplicate using the 8mm diameter Color Meter-Minolta 200b colorimeter. The apparatus was previously calibrated on a white surface according to the International Commission on Illumination – CIELAB – (Minolta, 1994).
Sensory evaluation
The study was approved by the UFSCar Ethics Committee under the CAAE: 32619014.5.0000.5504. The analysis was performed at the Sensory Evaluation Laboratory from UFSCar, Araras Campus. The tests were performed according to the Brazilian Association of Technical Standards - ABNT (1994), in individual booths under white light at 20°C. The samples were randomly coded with three digits and served simultaneously.
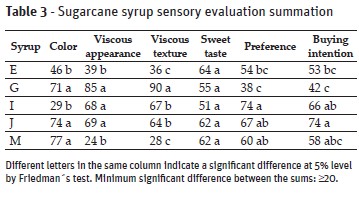
Five brands of sugarcane syrup (E, G, I, J and M) were selected from the values obtained in the instrumental analyzes of luminosity (L*) and soluble solids (°Brix), being chosen those that represented the extremes and midpoints of such attributes, so that the samples were representative of the total. It was applied the ranking test for difference and preference (ABNT, 1994) to evaluate the color (light-dark), visual viscosity (little-much), viscous texture (little-much) and sweet taste (minor-major), preference (minor-major) and buying intention (little-much). Twenty evaluators participated and 5mL of each syrup was served in coded transparent plastic cups, with a teaspoon, and the evaluators were asked to rank the samples according to the provided scale. Water was used to clean the palate between the samples.
Statistical analysis
The data on physicochemical analysis were evaluated by analysis of variance (ANOVA), considering significant the differences between the averages by Tukey test (p≤0.05).
The results of the ranking test were evaluated by Friedman's test (p≤0.05), using the table of Christensen et al. (2006), to verify the existence of a significant difference between samples, and with 5 samples and 20 evaluators, the minimum significant difference between the sums equal to 20 was considered.
RESULTS AND DISCUSSION
During the research period, it was visually noted that B, F, K, L, M and N brands showed sucrose crystallization. Crystallization causes the sucrose to precipitate, being withdrawn from the solution and thus causing changes in the product. The mean values obtained for pH (Table 1) ranged from 3.9 to 5.7, with a mean of 4.7. For honey, Nascimento et al. (2016) had a mean pH of 3.9. Barreto et al. (2015) found for sugarcane syrup, except for the samples produced with citric acid or acid lime, pH values between 5.0 and 6.5. The pH value is an indication of the acid content present in the product. However, as in cane syrup as in honey, the acids predominantly present are the organic ones, weak acids, and in this way the acidity determination makes the quantification of present acids more realistic.
As for acidity, values between 0.44 and 0.97 were obtained, with a mean of 0.70. To calculate the total acidity was used the aconitic acid as equivalent, with three acids terminals, as it is the main acid in sugarcane. Until 2005, the maximum acidity allowed in sugarcane syrup solution was 10% (Brasil, 1978, 2005). Thus, for this attribute, all samples of cane syrup were within the standard. Barreto et al. (2015) found in cane syrup, except for samples produced with citric acid or acidic lime, acidity values between 2.1 and 5.8%. Although the Brazilian legislation was repealed (Brasil, 1978, 2005), no new standard value was determined for the acidity of the cane syrup, being evident the need of a revision of such legislation and methodologies used, considering that the maximum value allowed was greater than the double found in commercial vinegars, with averages 4.2 and 4.1% for acetic acid (Farinazzo et al., 2015; Soares et al., 2016).
The mean values for soluble solids ranged from 72.3 to 82.9 °Brix, averaging 78.7 °Brix (Table 1). Barreto et al. (2015) found values ranging from 71 to 82 °Brix. Only four of the samples analyzed in this study would fit under the penultimate legislation which, although repealed, stipulated that the value should be between 50 and 74 ° Brix (Brasil, 1978, 2005).
The low content required by Brazilian legislation up to 2005 (Brasil, 1978, 2005) may be due to the lower crystallization rate in products with lower levels of soluble solids, but, due to the lack of natural or added antimicrobials, the cane syrup is subject to contamination and deterioration, reducing shelf life and product quality.
In this way, it is necessary to discuss and include in the current legislation the purpose of using cane syrup, and the range of ideal values for different cases. For use in the industry it is of extreme importance that the syrup does not crystallize and maintain its viscosity, either for final product uniformity or for processes optimization, such as pumping, therefore a low content of soluble solids is desirable. However, for domestic consumption, the shelf life may be more relevant, considering that mediums with lower water activity are unfavorable to the growth of microorganisms, and the presence of the sucrose crystals can be eliminated by raising the temperature.
The Table 1 shows the relation between the °Brix and the viscosity – the lower the soluble solids content, the lower the viscosity of cane syrup. The decrease in viscosity is also related to the release of water molecules when the sucrose crystals formation occurs, and it becomes evident when comparing the low viscosities of L, M and N brands to the crystallization observed in these samples.
The mean values for kinematic viscosity ranged from 9 to 314 St, with 116 St as mean and, as expected, the values were proportional to the soluble solids content, and consequently to the total solids, since the sugar concentration in the cane syrup, besides the possible presence of sucrose crystals, affect the fluidity thereof.
Knowing the sugarcane syrup viscosity is relevant because it is directly linked to consumer preferences and industrial production of food whose composition includes this product. In addition, the viscosity may be an indicative of variations in soluble solids or moisture content, and may indicate microcrystals formation. No other published references including the cane syrup viscosity were found.
Regarding moisture and total solids, the minimum values were 9.0 and 73.9%, respectively, while the maximum values were 26.1 and 91.0% and means 18.1 and 81.9% (Table 1). By the previous legislation (Brasil, 1978) the maximum moisture content should be 25%, thus, only two samples did not demonstrate conformity (B and L), which is consistent with the low viscosity verified in these samples, factor enhanced by the formation of sucrose crystals in them, since there is release of water molecules during the crystallization process. The current legislation (Brasil, 2005) does not stipulate ideal values for product moisture.
Ren et al. (2010) found in honey the same tendency from the product water content quantification: the higher the moisture or added water, the lower the viscosity. Since the moisture is inversely proportional to the total solids content, their results corroborate with this study.
For reducing sugars, the mean minimum value was, in percentage, 9.0, while the maximum was 61.5, and mean 38.0%. The values found by Barreto et al. (2015) were between 14 and 40.1%. There is great variation in this parameter - for cane syrup where sucrose hydrolysis is not forced (by addition of acid and temperature rise or by enzymatic action in natural degradation), the values are naturally low and depend on several factors, such as soil treatment, sugarcane genotype, fertilization and harvest time (Tasso Junior et al., 2007; Caputo et al., 2008; Santos et al., 2011). The N brand showed the formation of large sucrose crystals after opening the package, which is consistent with the low content of reducing sugars found - only 9%, as well as the M brand, which also showed a formation of a layer of crystals. It was also possible to visualize a fast crystallization of the L mark, even with sealed packaging, with only 13% of reducing sugars. The organic samples B and F also presented low values of glucose and fructose - this may be due to the manufacturing process or characteristics of the raw material, for example the maturation point of the sugarcane. Although the sample K also presented sucrose crystals, it presented higher reducing sugar values compared to the other crystallized samples. It may be due to the different manufacture date, as this information was not presented in every sample.
The knowledge of such sugars is important considering their influence on viscosity and product shelf life, and the greater the glucose and fructose content compared to sucrose, the lower will be the syrup crystallization, and so the greater its lifespan. In addition, the crystals formation is undesirable for the food industry because, in addition to hampering homogenization and standardization, it can cause damage to the equipment due to the accumulation of such crystals.
In ash analysis, in percentage, a minimum value of 0.3 was obtained, a maximum of 3.8 and an average of 1.6. Until 2005, the allowed ash content was up to 6% (Brasil, 1978), and in this base all samples had adequate amounts of ash. However, current legislation does not determine the ideal ash value in the product (Brasil, 2005). Barreto et al. (2015) found results between 1.2 and 2.1%, while Unicamp (2011) determined that the mean content of ash in cane syrup is 1.3%.
The ash content is linked to the minerals present in the product. In other studies, the following ranges for commercial sugarcane syrups were found in mg.100g-1 sample: 1.2 to 7.5 Fe, 62 to 699 K, 4.6 to 155 P, 4.6 to 57 Na, 0.04 to 2.4 Cu, 18 to 139 Mg, 0.31 to 1.59 Zn, 0.39 to 2.8 Mn and 22 to 50 Ca (Nogueira et al., 2009), evidencing the great variation of mineral nutrients in commercial sugarcane syrups, making it difficult to elaborate the nutritional characterization of the product.
Furthermore, the iron, phosphorus, sodium and manganese contents are significantly higher in commercial cane syrup than in those made from sugarcane juice grinded in a stainless steel mill; for iron and in some cases for copper the increase may be due to contamination during the manufacture of this product, especially when used copper pot (Nogueira et al., 2009). According to TACO (Unicamp, 2011), cane syrup has, every 100 g, 102 mg of calcium and 115 mg of magnesium.
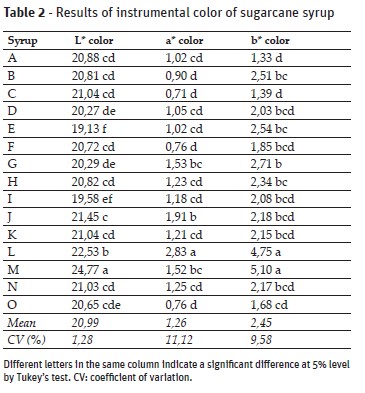
Changes in the coloration, brightness and saturation of the colors (Table 2) were recorded by means of the L* value (Luminosity), which ranges from black (L* = 0) to white (L* = 100); of the a* value, which characterizes coloration in the region of red (+a*) to green (-a*); and the b* value , which indicates the range from yellow (+b*) to blue (-b*) (Minolta, 1994).
The higher the luminosity index (L *), the lighter the cane syrup and, proportionally, as the index decreases the cane syrup color becomes darker. The minimum values found for luminosity, a* color and b* color were, respectively, 19.1, 0.7 and 1.3, while the maximum values were 24.8, 2.8 and 5.1, and the means 21.0, 1.2 and 2.4. The tonality is still a visual issue that may influence the consumer buying decision, as for pure cane syrup as for products made with it. No data previously published by other authors were found regarding sugarcane syrup color in the CIELAB scale.
When the 5 selected samples (E, G, I, J and M) were sensorially evaluated, the same difference between the visual color perception and its instrumental index was observed (Tables 2 and 3). The M brand, with higher value of instrumental luminosity, was perceived as the darkest by most evaluators. This is likely to be due to the small, dark-colored particles present in the syrup and sedimentation a few minutes after homogenization - so although the syrup was light in color, the particles altered the perception of it.
The evaluators were able to perceive the different viscosities in the same trend as the results found in the instrumental analysis, except for syrup I (Table 3). As in the physicochemical analysis, there was a relationship between the sensorial viscosity and the soluble solids content, with the same exception of syrup I. The sweet taste was not differentiated among the samples, although there was variation in soluble solids contents (°Brix).
Among the samples sensorially evaluated, the ones with the highest score for purchase intention and preference were those with a darker color and with a rather viscous appearance, but with low or moderate intensity viscous texture.
CONCLUSIONS
1.There is a great variation between the physicochemical parameters of sugarcane syrup, not necessarily implying a problem of technological quality;
2.The current legislation does not present acceptance ranges for the product characteristics, at the same time as the previous one is inadequate;
3.There was a direct relation between the soluble solids content and the instrumental and sensorial viscosities in 93% of the samples, but this was not related to sweet taste. It was also observed a relation between the instrumental and sensorial analyzes for color. There was greater purchase intention and preference for dark and visually viscous samples.
References
ABNT (1994) NBR 13170: Teste de ordenação em análise sensorial. Associação Brasileira De Normas Técnicas. Rio de Janeiro. 7p. [ Links ]
ABNT (2016) - NBR 16477: Líquidos usados em fundição – Determinação do tempo de escoamento pelo uso do copo Ford – Método de ensaio. Associação Brasileira De Normas Técnicas. 3p.
Barreto, P.P.P.; Bettani, S.R.; Borges, M.T.M.R. & Verruma-Bernardi, M.R. (2015) - Avaliação físico-química e sensorial de diferentes melados. Brazilian Journal of Agriculture, vol. 90, n. 3, p. 217-228. [ Links ]
Brasil (1978) - Resolução 12/33 de 1978 da Comissão Nacional de Normas e Padrões para Alimentos. Normatização brasileira relativa a açúcar mascavo, melado e rapadura. < http://www.anvisa.gov.br/anvisalegis/resol/12_78_melaco.htm>.
Brasil (2005) - Resolução 271 de 22 de setembro de 2005 da Agência Nacional de Vigilância Sanitária. Regulamento técnico para açúcares e produtos para adoçar. <http://portal.anvisa.gov.br/documents/33916/391619/RDC%2B271%2B-%2Bsetembro%2Bde%2B2005.pdf/c7b42446-fa36-4f04-a02d-29ad9bde032a>.
Caputo, M.M.; Beauclair, E.G.F; Silva, M.D.A. & Piedade, S.M.D.S. (2008) - Resposta de genótipos de cana-de-açúcar à aplicação de indutores de maturação. Bragantia, vol. 67, n. 1, p. 15-23. http://dx.doi.org/10.1590/S0006-87052008000100002 [ Links ]
Christensen, Z.T.; Ogden, L.V.; Dunn, M.L. & Eggett, D.L. (2006) - Multiple comparison procedures for analysis of ranked data. Journal of Food Science, vol. 71, n. 2, p. 132-143. https://doi.org/10.1111/j.1365-2621.2006.tb08916.x [ Links ]
De Sousa, A.; De Azevedo, E.; Eliete De Lima, E. & Da Silva, A.P.F. (2012) - Alimentos orgânicos e saúde humana: estudo sobre as controvérsias. Revista Panamericana De Salud Publica, vol. 31, n. 6, p. 513-517. [ Links ]
Eggleston, G. (2018) - Positive Aspects of Cane Sugar and Sugarcane Derived Products in Food and Nutrition. Journal of Agriculture and Food Chemistry, vol. 66, n. 16, p. 4007-4012. https://doi.org/10.1021/acs.jafc.7b05734 [ Links ]
Elmadfa, I. & Meyer, A.L. (2010) - Importance of food composition data to nutrition and public health. European Journal of Clinical Nutrition, vol. 64, n. 3, p. S4-S7. https://doi.org/10.1038/ejcn.2010.202 [ Links ]
Farinazzo, F.S.; Garcia, S. & Spinosa, W.A. (2015) - Caracterização de vinagre de fruta–maçã, a partir de frutos de cultivo orgânico e convencional. Blucher Biochemistry Proceedings, vol. 1, n. 2, p. 385-385. https://doi.org/10.5151/biochem-vsimbbtec-22208
ICUMSA (2011) - Method book. International Commission for Uniform Methods of Sugar Analysis. Berlin, Germany: Bartens. 128p. [ Links ]
IAL (2008) Normas analíticas do Instituto Adolfo Lutz: métodos físico-químicos para análise de alimentos. São Paulo: Instituto Adolfo Lutz, 1020 p. <http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf> [ Links ].
Jeronimo, E.M. (2018) - Produção de açúcar mascavo, rapadura e melado no âmbito da agricultura familiar e sua importância na alimentação humana. In: Magnoni Junior, L.; Stevens, D.; Purini, S.R.M.; Magnoni, M.G.M.; Vale, J.M.F.; Branco Junior, G.A.; Adorno Filho, E.F.; Da Silva, W.T.L. & Figueiredo, W.S. (Eds.) - Ciência alimentando o Brasil. 2ed. São Paulo: Centro Paula Souza. p. 111-120. [ Links ]
Liang, R. (2016) - Predicting intentions to purchase organic food : the moderating effects of organic food prices. British Food Journal, vol. 118, n. 1, p. 183–199. https://doi.org/10.1108/BFJ-06-2015-0215
Madail, J.C.M.; Belarmino, L.C. & Bini, D.A. (2011) - Evolução da produção e mercado de produtos orgânicos no Brasil e no mundo. Revista Científica da AJES, vol. 2, n. 3. [ Links ]
Minolta (1994) - Precise color communication: color control from feeling to instrumentation. Ramsey, 49 p. [ Links ]
Moratoya, E.E.; Carvalhaes, G.C.; Wander, A.E. & Almeida, L.M.D.M.C. (2013) - Mudanças no padrão de consumo alimentar no Brasil e no mundo. Revista de Política Agrícola, vol. 22, n. 1, p. 72-84, [ Links ].
Nascimento, L.O.; Pereira, J.R.; De Assis, C.L.; Alves, M.T.; Silva, P.S.; Fabri, R.L.; Mendonça, L.M.; Da Silva, J.G. & Denadai, A.M. (2016) - Comportamento térmico-reológico de xaropes compostos por mel e extratos naturais. Boletim do Centro de Pesquisa de Processamento de Alimentos, vol. 34, n. 2, p. 1-12. http://dx.doi.org/10.5380/cep.v34i2.53137 [ Links ]
Nogueira, F.S.; Ferreira, K.S.; Carneiro Junior, J.B. & Passoni, L.C. (2009) - Minerais em melados e em caldos de cana. Ciência e Tecnologia de Alimentos, vol. 29, n. 4, p. 727-731. [ Links ]
Ren, Z.; Bian, X.; Lin, L.; Bai, Y. & Wang, W. (2010) - Viscosity and melt fragility in honey–water mixtures. Journal of Food Engineering, vol. 100, n. 4, p. 705-710. https://doi.org/10.1016/j.jfoodeng.2010.06.004
Santos, D.H.; Silva, M.D.A.; Tiritan, C.S.; Foloni, J.S. & Echer, F.R. (2011) - Qualidade tecnológica da cana-de-açúcar sob adubação com torta de filtro enriquecida com fosfato solúvel. Revista Brasileira de Engenharia Agrícola e Ambiental, vol. 15, n. 5, p. 1807-1829. http://dx.doi.org/10.1590/S1415-43662011000500002 [ Links ]
Soares, D.M.; Nunes, J.A.; Cunha, S.F.; Cordeiro, T.C. & Alves, R.D. (2016) Determinação da concentração de ácido acético em vinagre comercial por volumetria ácido-base. Revista Educação, Meio Ambiente e Saúde, vol. 6, n. 4, p. 25-27. [ Links ]
Stupiello, J.M. (1987) – A cana-de-açúcar como matéria-prima. In: Paranhos, S.B. (Ed.) – Cana-de-açúcar: cultivo e utilização. Campinas, Fundação Cargill, p.759-804.
Sulzbacher, A.W. & David, C. (2009) - Agroindústria familiar rural: uma estratégia para melhorar a qualidade de vida no espaço rural. Geosul, vol. 24, n. 47, p. 69-90. [ Links ]
Tasso Júnior, L.C.; Marques, M.O.; Franco, A.; Nogueira, G.D.A.; Nobile, F.O.D.; Camilotti, F. & Silva, A.R.D. (2007) - Produtividade e qualidade de cana-de-açúcar cultivada em solo tratado com lodo de esgoto, vinhaça e adubos minerais. Engenharia Agrícola, vol. 27, p. 276-283. [ Links ]
Unicamp (2011) Tabela brasileira de composição de alimentos – TACO. 4.ed. Campinas: UNICAMP / NEPA. 161p.
Acknowledgement
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, for the scholarship granted during the period of this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Received/recebido: 2019.02.28
Accepted/aceite: 2019.04.05