INTRODUCTION
Crambe (Crambe abssynica Hoechst) is a crop that stands out due to the high oil content of its seeds and is currently studied in Brazil for biodiesel production. Crambe is a rustic Brassicaceae plant, easy to adapt to eutrophic soils, tolerant to droughts and frost. In addition to the low production cost, its cultivation is completely mechanized, made with the same machines used for small grains, which normally become idle during the winter, in the cerrado (Reginato et al., 2013).
The crambe seed contains an oil content greater than 35%, which consists of up to 57% erucic acid, a long chain fatty acid with high industrial value. Due to the presence of this acid, crambe oil it is not suitable for food; therefore, this culture is intended for industrial purposes only, not competing directly with the food supply (Carlson et al., 2007). It is a very promising crop for industrial uses; the oil from its seeds can be used in production of biofuels, biodegradable plastics, nylon, adhesives, electrical isolation, lubricants, anticorrosive agents, rubbers, and cosmetics (Falasca et al., 2010; Tavares et al., 2017).
Furthermore, the bran produced in the oil extraction process can be used for animal feed (Falasca et al., 2010; Goes et al., 2018), and the leaves used in the preparation of nematicides (Coltro-Roncato et al., 2016). Colodetti et al. (2012) cite the importance of crambe in biodiesel production; most of the oils used for this purpose come from annual crops, especially those with spring and summer cycles, with no other options available that allows the continuity of biodiesel production and use in industry.
In the crop establishment, the seed quality is a primordial factor. Seeds of low quality originate crops with inadequate population of plants, implying instability and economic loss for the producer (Krzyzanowski et al., 1993). In this sense, the establishment of crambe cultivation areas mainly depends on the development of suitable production technologies, with emphasis on the use of quality seeds.
Physiological quality relates to the seed ability to perform its vital functions, characterized by longevity, germination and vigor. Therefore, the effects on quality are usually translated by the decrease in the germination by the increase of abnormal seedlings and by the reduction of the vigor of the seedlings (Toledo et al., 2009).
In the areas of seed production, drying is an operation of fundamental importance, it is consequently a requirement to guarantee seed quality. Drying allows obtaining better quality seeds by making it possible to harvest in advance and to avoid damages that occur in the field due to climatic conditions, insect attacks and microorganisms as well as by decreasing the water content to safe levels, reducing the rate of seed deterioration during storage (Carvalho and Nakagawa, 2012).
In most crops propagated by seeds, such as crambe, the harvesting season does not coincide with the most suitable time for sowing, hence the importance of storing the seeds. During storage, the seeds are subject to the deterioration process, which leads to degenerative changes such as the destabilization of enzyme activity and the disruption and loss of integrity of the cell membrane system (McDonald, 1999).
Temperature and water content of the seeds are the most important factors for its feasibility preservation. For orthodox seeds, the best conditions for maintaining quality are low relative humidity and low temperature for reducing the metabolic activity of the embryo and, consequently, the deterioration (Marcos Filho, 2015). The deterioration process is inevitable; however, it can be delayed depending on the storage conditions and the seed characteristics.
Oilseeds have less storage potential than the amylaceous ones, considering the lower chemical stability of lipids compared to starch. A moderate temperature increase the respiratory process beeing enough to decompose lipids and, consequently, to elevate the deterioration rate (Fanan et al., 2009).
Studies on crambe culture are still incipient and basic information on high-quality seed production is, in some instances, contradictory. The aim of this work was therefore to assess the physiological quality of cramble seeds after drying at different temperatures and submitted to storage.
MATERIAL AND METHODS
The experiment took place at the Laboratory of Seed Research of the Department of Plant Science of the Federal University of Viçosa (UFV), Viçosa, Minas Gerais, Brazil. Seeds of crambe, ‘FMS Brilhante’ cultivar, from experimental area in the region of Viçosa, were harvested when the field had plants with 80% brown fruits.
Randomized blocks were the experimental design with four replications. The treatments were arranged in a 4x4 factorial scheme and consisted of four drying temperatures (natural and artificial air at 30, 45 and 60 °C) and four storage periods (0, 4, 8 and 12 months).
At the time of harvest, the plants were cut and taken to the laboratory, to have the racemes cut. The seeds were manually extracted and treated for the removal of barks and impurities. Subsequently, seed samples were submitted to drying with natural air (ambient with natural air circulation) and artificial drying (with drying air at 30, 45 and 60 ºC) until reaching 10% of water content.
After drying, the seeds were packed in paper bags (1 kg regular paper bag) and stored in an air-conditioned room for 12 months with an average temperature of 20 °C and relative humidity close to 55%. At the beginning of storage and every 120 days, the seeds were submitted to the following tests and/or determinations:
Water content: was determined according to the recommendations of the Rules for Seed Analysis (RAS) (Brasil, 2009), using the greenhouse method at 105 ± 3 °C for 24-h with results expressed as percentage.
Germination: was tested in gearbox plastic boxes, with three subsamples of 50 seeds per replicate. The seeds were distributed between two sheets of blotting paper, previously moistened with KNO3 solution in a volume equivalent to 2.5 times the weight of the dry paper. The boxes containing the seeds were placed in a B.O.D. germination chamber, regulated at a constant temperature of 25 oC, with constant light. Evaluations were performedon the fourth and seventh day after sowing, counting the number of normal seedlings, with the results expressed as a percentage, according to the criteria established by the RAS (Brasil, 2009).
First germination counting: conducted along with the germination test; it consisted on counting the number of normal seedlings on the fourth day after sowing (Brasil, 2009). The result is as percentage of normal seedlings.
Seedling emergence: was conducted under uncontrolled greenhouse conditions, with sand substrate washed and sterilized in an oven at 200 °C for two hours. The seeds were sown 3 cm deep in plastic trays and the substrate was moistened with water equivalent to 60% of the retention capacity, keeping it in this condition with daily light irrigations (Brasil, 2009). The number of normal seedlings on the seventh day after sowing were counted, and the results expressed as percentage of emergence of seedlings.
Electrical conductivity: was conducted with three subsamples of 50 seeds per replicate, weighed and packed in 200 mL disposable plastic cups containing 75 mL of distilled water. The cups were taken to the B.O.D chamber, set at 25 °C, and the readings occurred after 24-h of soaking, using a conductivity meter (DIGIMED DM 31) with results expressed in μS cm -1 g-1.
Data were tested for normality and homogeneity of variances and then submitted to analysis of variance. Tukey test, at 5% probability, was used to study the effects of the drying temperatures. The effects of the storage periods were studied by means of regression analysis, choosing the models that presented concomitantly higher determination coefficient (R2), estimates of the significant regression equation parameters at the level of 1 and 5% of significance by the “t test” and behavior with biological explanation.
RESULTS AND DISCUSSION
At the time of harvest, the seeds had a water content of 21.7%. After four months of storage, there was a small variation between the drying treatments in the water content of the seeds (6.0 to 7.2%), indicating homogeneity of the observed values. The water content of the seeds was within the maximum limit considered ideal for the storage of oilseed species, such as crambe. According to Harrington (1973), the ideal water content for storing seeds with high oil content is 9% (b.u.) and higher values lead to a rapid deterioration of these seeds.
During storage, the seeds had a hygroscopic equilibrium with the ambient air, since the water content of the seeds varied from 6.0 to 7.9%. The results were relatively low during the evaluated period, indicating that the water content should not have influenced the physiological potential of the seeds during the tests.
Seed preservation during storage is closely related to its water content, as this characteristic interferes with the chemical composition and speed of the metabolic activities of the seeds (Almeida et al., 2002). High water content contributes to the rapid loss of seed vigor depending on the storage conditions.
According to the analysis of variance there was a significant effect of drying and storage temperatures on all evaluated characteristics. The interaction between drying and storage temperatures was significant only for seed germination.
During storage, there was quadratic effect of drying with natural air, drying at 45 and 60 ºC in the germination of the seeds, while germination after drying at 30 ºC had a linear and increasing response behavior (Figure 1).
At the beginning of the storage, the seeds submitted to natural drying had 64.9% germination, with result increasing until the seven months of storage, when the maximum germination was verified (86.4%). These values indicate a 33.2% increase in germination of the seeds from the beginning of storage to the maximum point. From this point, there was reduction up to 12 months, observing 76.6% of germination (Figure 1).
At 30 ºC, the increases in germination occurred throughout the storage period, with a 34.6 % increase in seed germination until the end of storage. Seed germination was characterized by increases in the results, occurring until approximately the seven months of storage for drying seeds at 45 and 60 ºC (Figure 1), with germination reduction from this storage point.
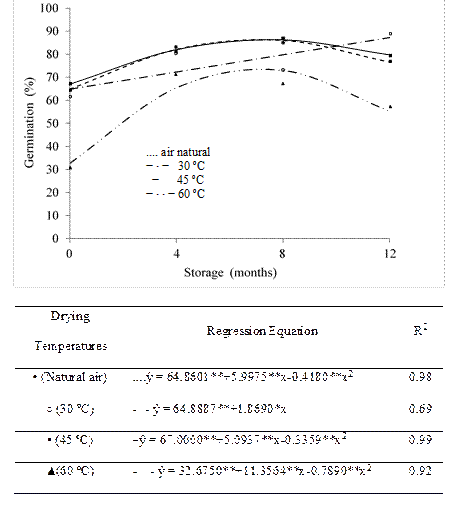
Figure 1 Seed germination (%) of crambe submitted to different drying temperatures during storage. ** and * significant at 1 and 5% by the "t" test, respectively.
Regardless of the drying temperature, the germination at the beginning of storage was lower than that observed at other storage times. Some researches indicate that the low germination presented by crambe seeds is due to some type of dormancy, as observed by Faria et al. (2014), which can be attributed to factors such as hardness of integument, hormonal regulation with excess of abscisic acid and absence of gibberellin, extreme temperature or intense cold and embryo immaturity (Martins et al., 2012). Costa et al. (2010) observed low germination values in freshly harvested crambe seeds, indicating seed dormancy. Costa et al. (2012) reported that crambe seeds showed dormancy at the beginning of storage. These authors emphasize the need to study dormancy in crambe seeds, considering that the germination capacity increases with the storage time.
Faria et al. (2012), studying the feasibility of freshly harvested crambe seeds submitted to different drying conditions and water contents, verified low germination and also seed dormancy. Amaro et al. (2015) reported that crambe seeds show post-harvest dormancy, which is partially overcome after six months of storage.
By analyzing the effects of drying temperatures on each storage period, drying at 60 °C was detrimental to the seeds at all evaluated storage times (Table 1).
Table 1 Seed germination (%) of crambe submitted to different drying temperatures during storage
| Drying Temperatures | Storage (months) | Means | |||
| 0 | 4 | 8 | 12 | ||
| Natural air | 64.5 a | 83.0 a | 85.0 a | 77.0 ab | 77.3 |
| 30 ºC | 62.0 a | 80.5 a | 73.0 b | 89.0 a | 76.1 |
| 45 ºC | 67.0 a | 81.0 a | 87.0 a | 79.5 ab | 78.6 |
| 60 ºC | 31.0 b | 71.0 b | 67.0 c | 57.0 c | 56.5 |
| Means | 56.1 | 78.8 | 78.0 | 75.6 | |
Means followed by different letters in the column differ from each other at 5% probability by the Tukey test.
Neves et al. (2007), studying two plots of crambe seeds submitted to two drying temperatures (25 and 30 ºC), found that the seeds had better performance when submitted to 30 ºC. However, they had low germination in all treatments, with 53% being the best result. Faria et al. (2014) concluded that drying temperatures above 60 °C act positively on the breakdown of dormancy of crambe seeds, with low water contents.
The milder drying temperatures (30 and 45 °C) were not detrimental to the physiological potential of the seeds, even in longer storage periods (Table 1). Reducing the water content from 21.7 to 10 % by drying up to 45 ºC did not damage seed germination at all storage times.
As shown in Figure 2, regardless of the post-harvest drying temperature, the results obtained at the beginning of storage were lower than those observed during storage, indicating that the conditions under which the seeds were stored were satisfactory in order to maintain their feasibility without loss of germination.
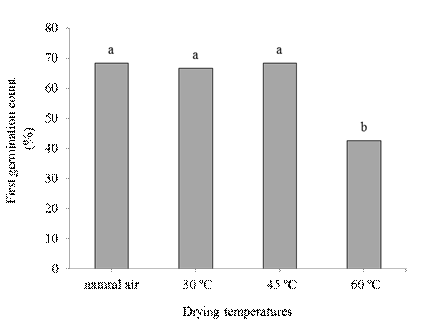
Figure 2 First seed germination count (%) of crambe submitted to different drying temperatures. Means followed by different letters in the columns differ among themselves at 5% probability by the Tukey test.
Marcos Filho (2015) reports that seed longevity varies according to genotype; however, the preservation period of the physiological potential depends to a large extent on the humidity degree and storage conditions. Orthodox seeds should be kept at a humidity content of 10% to 12 for storage for six to eight months; the lowest values are indicated for species in which lipid reserves predominate, such as crambe.
Drying temperatures and storage influenced independently when the first germination counting was evaluated. Drying seeds with air temperature at 60 °C was detrimental to normal seedlings emission (Figure 2) possibly due to cell membranes damage. There was no significant difference between drying with natural air at 30 and 45 ºC with values higher than 60% germination at the first test counting.
The first germination counting is a simple vigor test performed simultaneously on the germination test, and is based on the assumption that the most vigorous seeds will germinate faster than the others. Depending on environmental and drying conditions, there may be a reduction in the physiological quality of the seeds due to the intensification of the deterioration phenomena (Marcos Filho, 2015). Therefore, natural drying and drying with milder temperatures did not affect seed vigor.
The first germination counting data showed quadratic behavior during storage (Figure 3). In the initial evaluation, we verified the lowest values. There were increases in vigor up to eight months of storage with 72.1 % germination with reduction of values from that point.
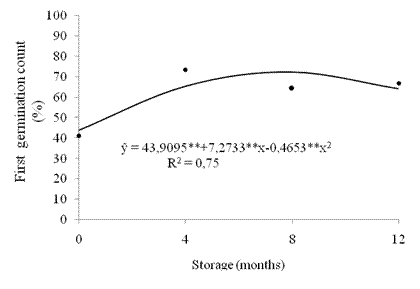
Figure 3 First seed germination counting (%) of crambe during storage. **significant at 1% by the "t" test.
Obtaining lower values from newly harvested seeds probably resulted from a manifestation of the residual dormancy of crambe seeds. This fact did not prevent germination but made the process slower and more uneven, which may have influenced vigor evaluation at the beginning of storage. According to Marcos Filho (2015), dormancy shows depth inversely proportional to its age, that is, it is more intense in freshly harvested seeds. According to Hilhorst (2007), differences in germination speed may be indicators of seed dormancy in different species and the time required for germination may vary with the dormancy degree.
Seedling emergence corroborated the results of the first germination counting, verifying independent effects of drying and storage temperatures on seed vigor. The lowest percentage of seedling emergence occurred at the highest drying temperature (60 ºC) with a value of less than 50% of emerged seedlings. In the seeds exposed to in natural air temperatures at 30 and 45 ºC, better performance occurred with results close to 60% seedling emergence (Figure 4). The results on seedling emergence test show that the use of high temperature provided detrimental effects on seed quality.
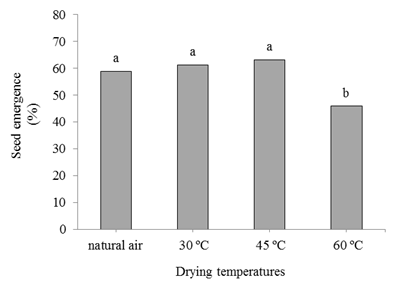
Figure 4 Seed emergence (%) of crambe from seeds submitted to different drying temperatures. Means followed by different letters in the columns differ among themselves at 5% probability by the Tukey test.
In a study with crambe seeds, Faria et al. (2014) concluded that the best performance in the physiological quality of seeds is obtained with water contents below 15%, dried at temperatures above 50 ºC. The authors point out that temperatures above 50 °C, associated with water contents above 15%, promoted significant damage to seed quality.
During the storage, seed vigor, which was evaluated by the seedling emergence test, was adjusted to a quadratic behavior model (Figure 5). At the beginning of storage, 54% of seedlings emerged. There were increases in the percentage of seedlings emerged up to five months of storage, reaching the maximum result of normal emerged seedlings (77 %). From this storage time, a great magnitude reduction occurred in the results. After 12 storage months, 30.8% of emerged seedlings were obtained, which indicates a pronounced fall in seed vigor.
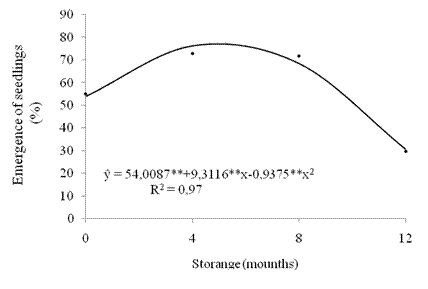
Figure 5 Emergence of seedlings (%) of crambe from seeds submitted to storage. **significant at 1% by the "t" test.
The manifestation of deterioration is closely associated with storage. However, it theoretically begins at physiological maturity, and can be accelerated at any post-maturity stage and may extend to the post-seeding period (Marcos Filho, 2015). The deterioration rate is influenced by the interaction between the biotic and abiotic factors. Regarding the environment, temperatures and high relative humidity accelerate deterioration during storage.
In this study, there were no significant variations of temperature and relative humidity of the air of the storage environment and the maintenance of the humidity level of the seeds remained at levels considered ideal for oilseed preservation. Thus, the reduction of seed vigor, as a deterioration manifestation, can certainly be attributed to the inherent characteristics of the species.
Seeds of oilseeds deteriorate due to lipid peroxidation. During storage, the conditions under which seeds are submitted are decisive for ensuring their physiological quality. Although this quality cannot be improved, adequate conditions during that period will help to maintain them viable for a longer time, delaying the deterioration process (Sediyama et al., 1981).
The electrical conductivity of the seeds was independently influenced by drying temperatures and storage. At 30 ºC, seeds showed the lowest mean electrical conductivity (Figure 6), that is, higher vigor. The other treatments provided the highest averages, indicating damage to cell membranes. As the natural drying did not differ from the other temperatures (45 and 60 ºC), it indicates that the time spent in natural drying was the cause of the harmful effect on the seeds.
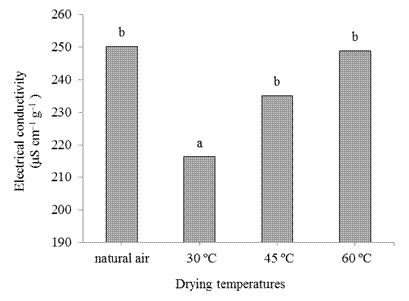
Figure 6 Electrical conductivity of seeds (μS cm-1 g-1) of crambe submitted to different drying temperatures. Means followed by different letters in the columns differ among themselves at 5% probability by the Tukey test.
Despite its advantages, drying is a potentially damaging process to seed quality and depends on the correct management of initial seed water content, temperature, relative humidity, airflow, drying rate and drying period of exposure to heated air (Miranda et al., 1999). Zonta et al. (2011) found that jatropha seeds (Jatropha curcas L., Euphorbiaceae) can be dried in the sun at 33 and 43 °C. They also verified that at 43 °C there were lower drying time, better germination and vigor of jatropha seeds, and shade drying was detrimental to seed quality.
The results of electrical conductivity of the seeds during storage were adjusted to a linear and increasing model (Figure 7). The release of seed exudates into the soaking water increased from the beginning of storage.
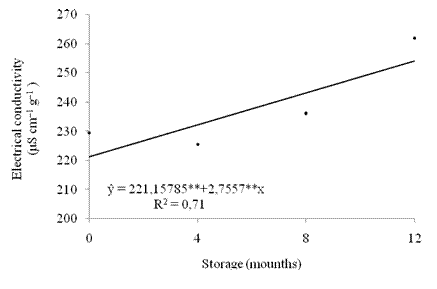
Figure 7 Electrical seed conductivity ((μS cm-1 g-1) of crambe during storage. **significant at 1% by the "t" test.
The electrical conductivity is a vigor test that evaluates the state of the cell walls by measuring the electrolytes in the water in which seeds were placed. The higher the conductivity value, the greater the seed damage (Krzyzanowski et al., 1999).
Considering that the water contents during seed storage did not reach harmful levels to their physiological quality (up to 9% for oilseeds according to Harrington, 1973), the decrease in quality is due to the natural seed deterioration processes, and as noted in this study, to the latent drying effects, since this is a process that occurs regardless of the conditions under which the seed is stored. Among the main changes involved in seed deterioration, the most important are reserves exhaustion; chemical composition alteration, for example, lipid oxidation and partial protein breakdown; cell membranes alteration, with integrity reduction and increase of permeability and disorganization; and enzymatic and nucleotide changes (Villela e Peres, 2004). The model proposed by Wilson and McDonald (1986) for orthodox seeds aging emphasizes that lipid peroxidation, as it results in the formation of free radicals, would be the primary cause of aging and loss of feasibility of the seeds during storage, which explains the high susceptibility of oilseeds to deterioration.
CONCLUSIONS
The seeds of crambe, ‘FMS Brilhante’ cultivar, showed post-harvest dormancy; however, it was completely overcome during storage.
Artificial drying at 30 and 45 °C does not affect the physiological performance of the seeds during storage and can be adopted in production systems of crambe seed.
Drying at 60 °C is detrimental to the quality of crambe seed, regardless of the storage period.
The physiological performance of the seeds decreases after eight months of storage.














