INTRODUCTION
Soil fungi are responsible for large losses in strategic crops with about 30% of diseases of emerging plants, being a threat to food security worldwide causing a loss of 125 million tons in different crops such as potatoes, wheat, soybeans, rice, and corn, also, causes an increase in the cost of production, as well as making certain planting areas unfeasible (Freire, 2015).
The genera Rhizoctonia, Fusarium, and Curvularia are among the main disease-causing fungi in plants (Correia and Michereff, 2018). The fungal species Rhizoctonia solani J.G. Kühn, causing seedling tipping and root rot, with a vast number of hosts (Lucon et al., 2009). The fungal species Fusarium oxysporum Schltdl. An inhabitant of the soil with resistance structures that attack the root system of the plant through natural lesions and colonizing the vessels and consequently causing the death of the plant (Medeiros et al., 2012). Another phytopathogen that has caused damage is the fungus Curvularia lunata (Wakker) Boedijn, a mitosporic fungi causing discoloration, lesions, and grain deformation mainly in rice (Oryza sativa L.) (Costa et al., 2017).
The chemical control of plant diseases is still widely applied, but due to the negative impacts on the environment, alternative searches for control of these phytopathogens are necessary (Silva et al., 2014a). The popularity of biological control has increased substantially since biological control is a sustainable, economical, and environmentally more attractive method than chemical control. Also, there are no phytotoxic effects on plants, besides not leaving toxic residues in plants and the environment (Bueno et al., 2015).
Within the alternatives studied, the use of antagonistic microorganisms is among the most sought-after tools currently in the integrated management of soil phytopathogens. Research advances in the search for more effective biological control agents, with the ability to adapt the same environmental conditions of phytopathogens, facilitating the development of these antagonists with the crops without causing damage and contributing to the promotion of plant growth (Fipke et al., 2017). Given this, biological products based on Trichoderma develop as an alternative to the use of chemicals, and they are considered with a low impact on the environment and healthier foods for the population (Junior et al., 2018).
In this context, the genus Trichoderma are free-living fungi, found most frequently in soils of temperate and tropical climate regions. Some species are used in the control of phytopathogens due to their polyvalence in action through a mechanism such as competition related to the interaction between two or more organisms, competing for air, light, water, and nutrients; host-parasite association characterized by a longer contact period, which may be physical or metabolic with digestion by hydrolytic enzymes, including chitinases, proteases, glucanases, and lipases; antibiosis being the action of one microorganism that acts on another, by releasing substances that harm or kill another microorganism (Machado et al., 2012; Machado and Silva, 2013).
In this context, the objective was to evaluate the in vitro antagonistic action of four species of Trichoderma in the pathogens R. solani, F. oxysporum, and C. lunata.
MATERIAL AND METHODS
Obtaining microorganisms
The species of Trichoderma were obtained by soil isolation from the experimental area of the Federal University of Tocantins (UFT), Gurupi Campus (11°43'45"S and 49°04'07"W, 300 m.a.s.l.) and in lowland areas of the municipality of Lagoa of Confusão - TO (10°47'37"S and 49°37'25"W, 200 m.a.s.l.). The soil samples were collected at a depth of up to 20 cm at random points in the areas mentioned and taken to the Laboratory of Agromicrobiology of UFT. Then, a sample (1 g) of each soil was deposited and scattered in a Petri (90x15 mm) dish containing PDA (potato-dextrose-agar- Kasvi, 27 g/L) culture medium modified with oxytetracycline hydrochloride (100 mg.L-1) (Terramycin®, Pfizer), to inhibit bacterial growth. Then the plates were incubated in a growth chamber, type Biochemical Oxygen Demand (BOD), at a temperature of 25°C ± 2°C with a photoperiod of 12 hours, for seven days.
Later in the laminar flow chapel part of the mycelium of the fungus with more aggressive growth characteristics and filamentous aspect of green colour, they were transferred to new Petri dishes with medium and again incubated. The peaking procedure was repeated to obtain the pure colonies of Trichoderma. Gender identifications were previously morphologically identified (Hunter, 1998; Zafari et al., 2004) and characterized by sequencing of the TEF region (Translation Elongation factor) and identified by the access codes in the GenBank carried out by the Biological Institute of São Paulo (Table 1). The isolates were kept in a refrigerator with peaking in PDA medium and preserved in water, according to Castellani methodology (Pires et al., 2012), for its better conservation. The species used were: T. harzianum Rifai (UFT-25), T. asperellum Samuels, Lieckf. & Nirenberg (UFT-205), T. virens (J.H. Mill., Giddens & AA. Foster) Arx (UFT-57), and T. longibrachiatum Rifai (UFT-204).
Table 1 GenBank access codes for isolated from Trichoderma (TEF) used in this study
| Isolated | Species identification | Access GenBank | Reference |
| UFT-25 | T. harzianum CIB T131 | EU279988 | Hoyos-Carvajal et al. (2009) |
| UFT-57 | T. virens CIB T147 | EU280060 | Hoyos-Carvajal et al. (2009) |
| UFT-204 | T. longibrachiatum DAOM 167674 | EU280046 | Hoyos-Carvajal et al. (2009) |
| UFT-205 | T. asperelloides GJS 04-217 | DQ381958 | Samuels et al. (2010) |
Obtaining pathogens
The pathogens were obtained from the Phytopathology Laboratory of UFT - Gurupi Campus. Fungi were obtained from soybean (Rhizoctonia solani and Fusarium oxysporum) and rice (Curvularia lunata) crops from the southern Tocantins with typical symptoms of the diseases described above and cultivated in PDA culture medium.
Antagonism test (Pairing)
Paired plate cultivation was performed, in which agar discs (5 mm in diameter) containing mycelium of each pathogen were placed in Petri dishes containing PDA medium, at a distance of approximately 1.0 cm from the edge. The plates were incubated for 24 hours in the absence of light. Subsequently, Trichoderma spp. were inoculated in the Petri dishes in a position opposite to the pathogen colony. For the control, 0.5 cm diameter discs of the phytopathogen and antagonist were transferred to the centre of isolated Petri dishes containing PDA medium, and the colonies were not paired. The plates were incubated in Biochemical Oxygen Demand (BOD) chamber at 28°C ± 2°C, with a photoperiod of 12 hours of light.
After seven days of colony pairing, the colonization percentage (%C) was evaluated according to Camporota (1985) methodology, in which: %C = DT/DE x 100, being DT the growth radius of the Trichoderma spp. colony in the frontal direction of the pathogen colony and DE the distance between the two colonies. Another evaluation by inhibition percentage = [(C - T)/C] x 100, where; C= radial control growth; T= radial treatment growth. Finally, an evaluation was also carried out according to the criteria proposed by Bell et al. (1982) with adaptations by Chagas (2015) (Table 2). It was considered the isolate with antagonistic or efficient action when its score is less than or equal to 2.0.
Table 2 The scale used for the crop pairing test was proposed by Bell et al. (1982) and adapted by Chagas (2015)
| Score | Evaluation scale | Colonization (%) |
|---|---|---|
| 1 | Antagonist grows all over the plate and over the disc of the pathogen | 87.5 to 100 |
| 1.5 | Antagonist grows over 7/8 of the dish | 66.6 to 87.5 |
| 2 | Antagonist grows over 2/3 of the dish | 62.5 to 66.5 |
| 2.5 | Antagonist grows about 5/8 of the dish | 51 to 62.4 |
| 3 | Antagonist and pathogen grow to half the dish | 50 |
| 3.5 | Antagonist grows over 3/8 of the dish | 37.5 to 49.9 |
| 4 | Antagonist grows over 1/3 of the dish | 33.3 to 37.4 |
| 5 | Antagonist does not grow in Petri dish | < 33.2 |
Assay with volatile metabolites
For the volatile metabolite test, the methodology proposed by Bonfim et al. (2010) was used, where discs containing mycelium of the pathogens Fusarium oxysporum, Curvularia lunata, and Rhizoctonia solani were inoculated separately in PDA medium, after 24 hours incubation bases of other Petri dishes of the corresponding size, containing PDA medium, received at their centre an air disc containing mycelium of the antagonists. The plates overlapped so that the antagonist was at the bottom and the pathogen in the upper part. The same plates were firmly sealed and incubated at 28ºC ± 2 ºC, with a photoperiod of 12 hours. The evaluation was performed on the 7th day.
To evaluate the inhibition by volatile metabolites, with the aid of a calliper, the diameter of the colonies (mm) was measured in three diametrically opposite directions in comparison to the growth of the control diameter that grew in the absence of the antagonist.
Statistical analysis
Both tests were performed in triplicates and the analysis in a 4x3 factorial scheme (4 isolates and 3 pathogens). The experimental design was completely randomized (IHD). The data obtained were submitted to the analysis of variance (ANOVA) and the means grouped by the Scott-Knott test at 5%, using the Software SISVAR, version 5.1 (Ferreira, 2000).
RESULTS AND DISCUSSION
Antagonism test (Pairing)
The different species of Trichoderma showed efficiency in the control of pathogens, ranging from 57.5% to 81.4% of colonization, 65.6% to 87.0% inhibition, and scores from 1.5 by the Bell et al. (1982) scale (Table 3). In the analysis of the individual potential of each antagonist in the control among pathogens, the isolate UFT-25 (Trichoderma harzianum) did not differ statistically in the percentage of colonization, only in the inhibition with the lower result to the pathogen Curvularia lunata (76.3%) (Table 3). The isolate UFT-205 (T. asperelloides) obtained better colonization with Fusarium oxysporum (80.8%) and C. lunata (81.4%) and did not differ in the percentage of inhibition among pathogens (Table 3). In the case of the isolate UFT-57 (T. virens), colonization did not differ among pathogens but obtained lower inhibition in pairing with C. lunata (72.0%). The isolate UFT-204 (T. longibrachiatum) was more efficient in colonization with the pathogens Rhizoctonia solani (73.7%) and C. lunata (69.0%) and better inhibition to the pathogen R. solani (82.0%) (Table 3).
Table 3 Classification of Trichoderma species for in vitro antagonism evaluated by colony pairing to pathogens Rhizoctonia solani, Fusarium oxysporum, and Curvularia lunata, according to colonization percentage (%C), inhibition percentage (%I), and Bell scale score
| Isolate | Rhizoctonia solani A | Fusarium oxysporum B | Curvularia lunata C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| %C | %I | Score | %C | %I | Score | %C | %I | Score | |
| UFT-25 | 76.7 aA | 84.6 aA | 1.5 | 73.3 bA | 87.0 aA | 1.5 | 77.0 aA | 76.3 aB | 1.5 |
| UFT-205 | 68.8 aB | 79.6 aA | 1.5 | 80.8 aA | 85.3 aA | 1.5 | 81.4 aA | 80.7 aA | 1.5 |
| UFT-57 | 73.7 aA | 81.7 aA | 1.5 | 70.5 bA | 84.2 aA | 1.5 | 76.0 aA | 72.0 bB | 1.5 |
| UFT-204 | 74.7 aA | 82.0 aA | 1.5 | 57.5 cB | 72.3 bB | 1.5 | 69.0 bA | 65.6 cC | 1.5 |
| Control | 100 bA | - | - | 100 dA | - | - | 100 cA | - | - |
| **C.V.1 % ***C.V.2 % | 5.56 3.92 | ||||||||
*Averages followed by the same lowercase letter in the column and uppercase in the row do not differ from each other by the Scott-Knott test (P<0.01).
**C.V.1 = Coefficient of variation (colonisation); ***C.V.2 = Coefficient of variation (inhibition).
In the confrontation with the pathogen R. solani, it was verified on the seventh day of incubation that Trichoderma isolates did not differ statistically, varying their colonization from 68.8% to 76.7% and inhibition from 79.6% to 84.5%. In the scores attributed by the criterion of Bell et al. (1982), all isolates obtained a score of 1.5, which corresponds to the growth on 7/8 of the plate (Figure 1, B2 to B4), 66.6% to 87.5% of the occupied area.
The isolate UFT-25 showed greater parasitism capacity, occurring growth and sporulation on the mycelium of R. solani paralyzing its growth or even possibly death (Figure 1, B1). The isolates UFT-57 and UFT-204, although they did not form an inhibition halo and overlapped the pathogen, were able to inhibit the growth of the pathogen (Figure 1, B3, and B4). Regarding the formation of halo (inhibition zone) to the pathogen, it was markedly observed by the isolate UFT-205 (Figure 1, B2) when physical contact occurred between the mycelium of Trichoderma and R. solani probably due to the release of toxic substances that inhibited the growth of the pathogen. Brito et al. (2010) in the confrontation of Trichoderma spp. against Sclerotinia sclerotiorum (Lib.) de Bary also found the formation of an inhibition halo. This fact supports the hypothesis that these isolates possibly produced volatile and non-volatile metabolites (antibiosis), which inhibited the pathogen's advancement. The ability to produce these volatile and non-volatile metabolites by isolates of Trichoderma spp. was reported by Lopes et al. (2012) and Dildey et al. (2014).
Sousa et al. (2017), evaluating the biocontrol potential of R. solani of cowpea by isolates of Trichoderma spp. found an inhibition reduction of 41.6% of the pathogen mycelial growth. The results obtained in the present study are more expressive since the inhibition of isolates reached 84.6% (Table 3). However, the degree of biocontrol of these antagonists may vary according to the isolates and their adaptation, within and among species, and present different behaviours about pathogens, due to the origin of the isolated fungus, nutritional status of the medium, and pH, and may influence the different mechanisms of fungal action and may act more aggressively in some cases (Dennis and Webster, 1971).
Regarding the confrontation with the pathogen F. oxysporum, the isolate UFT-205 obtained better colonization with 80.8% (Table 3). Regarding pathogen inhibition, isolates UFT-25, UFT-205, UFT-204 did not differ from each other, with a percentage of 87%, 85.3%, and 84.2%, respectively, being higher than the UFT-57 isolate. According to the evaluation criteria by Bell et al. (1982), all isolates in the confrontation with F. oxysporum received a score of 1.5, which represents a growth of 7/8 of the plate (Figure 1 D1 to D4). In the pairing of the antagonist with F. oxysporum, it showed that the different species of Trichoderma may have presented different control mechanisms such as parasitism, predation, or antibiosis. The antagonistic activity of Trichoderma spp. is usually associated with hydrolytic enzymes such as chitinases and glucanases expressed in Trichoderma spp. interactions with phytopathogenic fungi and antibiotic production, such as viridine, gliotoxin suzucacilin, trichodermin, and trichothecene, showing a joint work with degrading enzymes of the cell wall, to potentiate the control of pathogens (Kubicek et al., 2011; Tijerino et al., 2011).
The inhibition halo in the confrontation with F. oxysporum is shown by the isolates UFT-25 and UFT-205 (Figure 1, D1 and D2), also growth and sporulation occurred on the pathogen colony, being more aggressive than the isolate UFT-204 (Figure 1, D4). Lima et al. (2016) also observed a strong inhibition in the growth of Fusarium spp. and Bipolares spp. in confrontation with Trichoderma spp. in which it occupied 66.6% (44.4% for Fusarium spp.) at 100% of the area dish, overlapping the pathogen colony.
Similar results were also reported by Silva et al. (2014b) in the pairing of Trichoderma spp. isolated from passion fruit on the mycelial growth of different isolates of F. solani. It was observed that at 12 days of incubation the antagonist isolates grew on the pathogen, covering the entire surface of the medium. A plausible explanation for the relevance of the results obtained may be the interaction between the different mechanisms of action (antibiosis, parasitism, predation, and competition) of Trichoderma.
Our results differ from those reported by Mendes et al. (2018), where they evaluated two species of Trichoderma (T. harzianum and T. longibrachiatum) for the inhibition capacity to F. oxysporum. T. longibrachiatum showed higher potential for F. oxysporum inhibition when compared to T. harzianum, inhibiting 71.2% and 69.7%, respectively. In the present study, the percentage of inhibition for T. harzianum (87%) and T. longibrachiatum (72.3%) was higher than the values observed by the previously cited authors. This variation in the results may be related to factors reported by Raimundo et al. (2016), which evaluated the efficiency of T. harzianum and T. asperellum Samuels, Lieckf. & Nirenberg as antagonists to Fusarium fujikuroi Nirenberg (=F. verticillioides (Sacc.). The authors emphasized that the action of the antagonist may vary depending on the pathogen species, the antagonist, and incubation conditions, and thus each mechanism express its potential in parasitism, predation, or antibiosis.
In the confrontation with the pathogen C. lunata, the isolates UFT-25, UFT-205, and UFT-57 obtained the highest percentage of colonization, when compared with the other studied pathogens, with values varying from 76% to 81.4% (Table 3). Regarding inhibition of pathogen growth, isolates UFT-25 and UFT-205 showed higher inhibitory values of the pathogen with 80.7% and 76.3%, respectively (Table 3). In the scores attributed by the criterion of Bell et al. (1982), the isolates obtained a score of 1.5, which corresponds to the growth on 7/8 of the plate (Figure 1, F1 to F4), 66.6% to 87.5% of the occupied area.
The present study demonstrated the efficacy of Trichoderma isolates against the pathogen C. lunata in pairing test, the isolates inhibited mycelial growth, forming inhibition halo (Figure 1, F1, and F3). An indication of phytopathogen growth suppression is due to the production of non-volatile metabolites (antibiosis). Baiyee et al. (2019) obtained satisfactory results evaluating the efficacy of T. spirale in the control of C. lunata with inhibition of 85.6% to 93,0%. Also, according to the authors, several species of Trichoderma produce hydrolytic enzymes responsible for the degradation of the fungal cell wall. Β-1,3-glucanase and chitinase produced by Trichoderma species are the main enzymes of cell wall degradation. Fungus of the genus Trichoderma has been used in several cultures as biocontrol, due to its ability to compete for nutrients and space, antibiosis, and parasitism, besides promoting plant growth and defence mechanisms (Haddad et al., 2017; Carvalho et al., 2018).
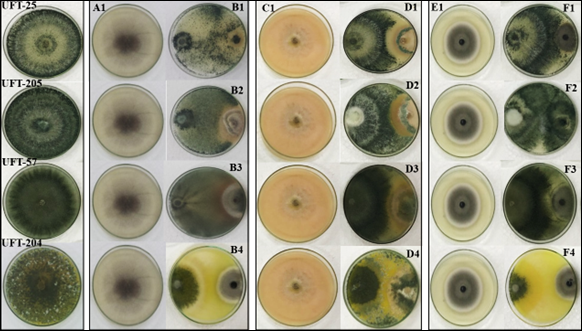
Figure 1 Confrontation of Trichoderma isolates with the pathogens Rhizoctonia solani (B1 to B4: right side pathogen, antagonist left side), Fusarium oxysporum (D1 to D4: right side pathogen, antagonist left side), and Curvularia lunata (F1 to F4: right side pathogen, left side antagonist). A1 - Control R. solani; C1 - Control F. oxysporum; E1 - Control C. lunata. B1=Confrontation A1xUFT-25; B2=Confrontation A1xUFT-205; B3=Confrontation A1xUFT-57; B4=Confrontation A1xUFT-204. D1=Confrontation C1xUFT-25; D2-Confrontation C1xUFT-205; D3=Confrontation C1xUFT-57; D4=Confrontation C1xUFT-204; F1=Confrontation E1xUFT-25; F2=Confrontation E1xUFT-205; F3=Confrontation E1xUFT-57; F4=Confrontation E1xUFT204.
Assay with volatile metabolites
The efficiency of the production of volatile secondary metabolites was verified by the biological activity of Trichoderma isolates on the phytopathogens R. solani, F. oxysporum, and C. lunata where antifungal action with reduction of mycelial growth of pathogens was observed with values ranging from 81.4% to 57.5% (Table 4). In the evaluation of each isolate, UFT-25 and UFT-57 did not differ among pathogens. The isolate UFT-205 was more efficient in the control of R. solani (67.7%) (Table 4). Regarding the isolate UFT-204, the greatest reduction occurred to the pathogen F. oxysporum (57.5%).
Table 4 Classification of Trichoderma species for in vitro antagonism evaluated by volatile metabolites to the pathogens Rhizoctonia solani, Fusarium oxysporum, Curvularia lunata*
| Isolate | Rhizoctonia solani | Fusarium oxysporum | Curvularia lunata |
|---|---|---|---|
| Reduction of pathogen mycelial growth (%) | |||
| UFT-25 | 76.7 aA | 73.3 bA | 77.0 bA |
| UFT-205 | 67.7 aA | 80.8 cB | 81.4 bB |
| UFT-57 | 73.7 aA | 70.4 bA | 76.0 bA |
| UFT-204 | 74.7 aB | 57.5 aA | 69.0 aB |
| Control | 100.0 bA | 100.0 dA | 100.0 cA |
| **C.V. % | 4.5 | ||
*Averages followed by the same lowercase letter in the columns and uppercase in the rows did not differ statistically from each other by the Scott-Knott test (P<0.01). **C.V. = Coefficient of variation.
Comparing the results of mycelial growth of R. solani in the presence and absence of volatile metabolites produced by the antagonist, it was observed that the isolates did not differ in the degree of inhibition, reducing the pathogen mycelial growth from 23.3% to 32.5% compared with the control colony of the pathogen (Table 4). Regarding F. oxysporum, the isolate UFT-204 provided the greatest reduction in mycelial growth of the pathogen in 42.5% compared to the control. For biocontrol of the pathogen C. lunata, the isolate UFT-204 showed more efficient values with 31.0% of mycelial reduction of the pathogen colony.
This reduction in the mycelial growth of pathogens probably occurred due to the ability of these antagonists to produce volatile compounds that significantly interfere with the growth of pathogens, demonstrating the isolate's antagonistic potential. There is a variability of volatile compounds produced in Trichoderma species: 6-pentilpiran-2na (6PP), 3,4-dimethyl-1-hydroxybutanoic acid, 2-methylpropan-1-ol, oct-1-en-3-ol, octan-3-one, octan-3-ol, and oct-1-en-3-ona are examples of secondary metabolism products produced by fungi of these genera that exhibit fungistatic and fungicide effects being important in the microbial kingdom. Also, the volatile metabolites produced by Trichoderma spp. play a primary role in mycoparasitism and its interactions with plants (Dias, 2014).
In the present study, the antagonistic activity by volatile compounds of Trichoderma in reducing the growth of the pathogen ranged from 18.6% to 42.5% (Table 4). The ability of the fungus of this genus to inhibit the growth of pathogens was observed by Broetto et al. (2014) in the evaluation of several species against Macrophomina phaseolina (Tassi) Goid. Their results are within the inhibition range found in our study, as inhibition capacity reached 23.1% by the isolate TLB12 (T. harzianum). The results obtained by Mesquita et al. (2017) are also followed in the present study, in which they found an inhibiting effect of volatile metabolites of 13 isolates of Trichoderma to the pathogen S. sclerotiorum, of which 12 presented similar action with a reduction in mycelial growth of 39.5% to 13.3%.
The results of the present study suggest that the different species of Trichoderma can be explored in the control of the pathogens R. solani, F. oxysporum, and C. lunata. However, it is suggested to carry out tests to assess the antagonistic potential in vivo to achieve greater efficiency in the control and to be adopted in the integrated management of diseases.
CONCLUSIONS
The in vitro trials showed the antagonistic action of Trichoderma isolates on the growth of the pathogens Fusarium oxysporum, Curvularia lunata, and Rhizoctonia solani. In colony pairing, the isolate UFT-205 (T. asperelloides) has greater potential in the biocontrol of F. oxysporum, presenting higher colonization (80.8%) and inhibition (85.3%). The isolates UFT-25 (T. harzianum) and UFT-205 (T. asperelloides) better control the pathogen C. lunata, with better colonization results (77% to 76.3%) inhibition (80.7% to 81.4%). All Trichoderma isolates were efficient in the control of R. solani, with colonization of 68.8% to 76.7% and inhibition from 79.6% to 84.6%. It was verified, according to the Bell scale, that all isolates are efficient antagonists with a score of 1.5.
Trichoderma isolates inhibited the mycelial growth of the plant pathogens studied. The isolate UFT-204 (T. longibrachiatum) provided a greater reduction in mycelial growth of the pathogens F. oxysporum (42.5%) and C. lunata (31%). For the pathogen Rhizoctonia solani, all isolates of Trichoderma reduced (23.3% to 32.3%) the plant pathogens colonies.














