INTRODUCTION
Polycyclic Aromatic Hydrocarbons (PAHs) are chemical substances characterized by two or more fused benzene rings. The aromatic structure of these rings gives rise to very stable compounds, so, they show a low degradability and high persistence in the environment, being considered priority pollutants.
PAHs can be grouped in Low Molecular Weight PAHs (LMW, 2-3 rings) and High Molecular Weight PAHs (HMW, 4 or more rings), showing different properties. LMW PAHs are easier to be dissipated from soils by volatilization or biodegradation, contrary to HMW PAHs, which are more recalcitrant to microbial attack (Leech et al., 2020). This different behavior affects the capacity to remediate PAHs polluted soils. In order to increase their bioavailability for soil microorganisms, extractants and solubilizing agents are used, and non-toxic solubility enhancers such as cyclodextrins (CDs) and biosurfactants (BSs) have been increasingly used in the last years.
In the present study, a 120-days incubation in microcosm experiment was conducted on three soils with different properties, artificially contaminated with 14 PAHs (from 3 to 6 rings) to investigate the effect on their dissipation of: i) the soil properties; ii) three availability enhancers (two CDs and a rhamnolipid BS).
MATERIALS AND METHODS
Soils
Three agricultural soils (CR, CN and TM) of different textures and properties were selected. They were sampled from different areas of Southwest Spain showing a background of several pesticides application. Soils were sampled from the 0-10 cm layer, dried at room temperature, sieved by 2 mm, and stored at 4°C. Physicochemical characteristics are shown in Table 1. The three soils were selected because they showed in preliminary tests a microbial flora capable of provoking the natural attenuation of pyrene when they were artificially contaminated (data not shown).
Incubation assay
Incubation experiments were carried out in glass containers (300 mL) in triplicate for each treatment. The 3 soils were spiked with a mix of 14 PAHs (40 mg kg-1 ∑14PAHs from 3 to 6 rings; (acenaphthylene (ACL), acenaphthene (ACE), anthracene (ANT), fluorene (FLU), phenanthrene (PHE), fluoranthene (FLT), pyrene (PYR), benzo[a]anthracene (BANT), chrysene (CHR), benzo[a]pyrene (BPYR), benzo[b]fluoranthene (BFLT), dibenzo[a,h]anthracene (DBANT), benzo [g,h,i]perylene (BPER), and indeno[1,2,3-c,d]pyrene (IPYR)). Two CDs (2-hydroxypropyl-β-cyclodextrin (HP) and randomly methylated-β-cyclodextrin (RAMEB)) and a rhamnolipid (RL) were used as availability enhancers at a dose of 1% (dry soil basis). Samples were taken at 1, 15, 30, 60 and 120 days for individual PAHs content analysis.
Statistical analysis
Data of the 14 PAHs sudied contents at every incubation time were statistically analysed using IBM SPSS Statistics v.25. Varimax-rotated principal components analysis (PCA) was used to ascertain the existence of possible associations among variables (PAHs contents) in order to get a dimensionality reduction associated to the evolution of the 14 PAHs studied affected by the 3 experimental factors: soil (CR, CN, TM), availability enhancer (control, HP, RAMEB, RL) and incubation time (1, 15, 30, 60, 120 days)).
RESULTS AND DISCUSSION
Natural attenuation of PAHs in all soils showed high degradation capacity for seven of the lower molecular weight PAHs (ACL, ACE, ANT, FLU, PHE, FLT, and PYR), with a final content < 5% of their initial concentration. Conversely, for the rest of the PAHs (high molecular weight PAHs, HMW) biodegradation is considered almost negligible, remaining in the soils (from 61% - 83.5%), with a percentage of abiotic dissipation directly related with the OM content of the soils, due to formation of non-extractable residues.
PCA is a useful tool for treating a large amount of data, as in this assay. For this analysis, individual PAH contents at every incubation time and treatment have been included. PCA grouped the PAHs into three principal components (PCs) that explain 82.0% of the total variance observed (Table 2).
Table 2 Correlation coefficients between the content of PAHs and the first three principal components resulting from PCA, eigenvalues and percentage of variance accounted for
| PAH | Component | |||
| PC1 | PC2 | PC3 | Communalities | |
| FLU | 0.914 | 0.044 | 0.097 | 0.846 |
| ACE | 0.889 | 0.087 | -0.133 | 0.815 |
| FLT | 0.872 | 0.205 | 0.173 | 0.833 |
| ANT | 0.858 | -0.020 | 0.250 | 0.798 |
| ACL | 0.842 | 0.057 | -0.104 | 0.722 |
| PYR | 0.818 | 0.168 | 0.125 | 0.713 |
| PHE | 0.803 | 0.374 | -0.212 | 0.830 |
| IND | 0.065 | 0.958 | 0.033 | 0.922 |
| DBANT | 0.005 | 0.890 | 0.238 | 0.848 |
| BPER | 0.060 | 0.877 | 0.049 | 0.776 |
| BFLT | 0.211 | 0.775 | 0.138 | 0.664 |
| BPYR | 0.556 | 0.719 | -0.067 | 0.831 |
| CHR | -0.100 | 0.070 | 0.959 | 0.934 |
| BANT | 0.229 | 0.255 | 0.909 | 0.944 |
| Eigenvalue | 5.567 | 3.890 | 2.020 | |
| Variance (%) | 39.764 | 27.784 | 14.427 | |
| Cumulative | 39.764 | 67.548 | 81.975 | |
The first component (PC1) explained 39.8% of the variance and was associated with 3-rings PAHs (FLU, ACE, ANT, ACL, and PHE) and two 4-rings PAHs (FLT and PYR). In this way, among the PAHs studied, PC1 has grouped those 7 with log Kow ≤ 5.2 which have shown higher dissipation rates (Madrid et al., 2021). This means that, probably, their dissipation processes were affected by similar factors. All other PAHs included in the analysis have log Kow ≥ 5.6 and were mainly associated to the other PCs. PC2 explained 27.8 % of the variance and grouped the heavier PAHs, those that have 5- (BFLT, BPYR, and DBANT) or 6-rings (IPYR and BPER) and log Kow ≥ 6.2. This could be related with the high adsorption capacity of these PAHs. In this group of PAHs, BPYR showed a lower correlation value with PC2 and a higher value with PC1 than the others, probably because of the moderate dissipation that this PAH showed in the 3 soils. On the contrary, the other PAHs grouped by PC2 have shown very low dissipation rate after 120 days. Finally, the third Principal Component extracted (PC3) explains 14.4% of the variance and directly groups the other 4-rings PAHs not included in PC1, CHR and BANT. Their behaviors have been more erratic than PYR and FLT, probably related with the higher log Kow. Golobočanin et al. (2004) applied PCA analysis to a wide set of PAHs contaminated soils. These authors also found an influence of the number of rings in the distribution of the different PAHs among the extracted components.
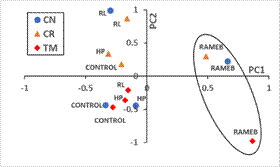
To describe the global effect of the availability enhancers used for every soil, Figure 1 shows the mean scores of each treatment plotted on the plane PC1 vs. PC2. It is observed that RAMEB treatments (that retarded the dissipation of PAHs, Madrid et al. 2021) showed the highest values in PC1 for the three soils. Other treatments (HP, RL, and control), with higher dissipation rates of the lighter 7-PAHs, show negative mean scores in this component. In summary, lower values in PC1 is related with decreasing PAH content along the incubation period.
It is corroborated in Figure 2 representing the evolution of the centroid values at every incubation time for the three soils and for all treatments simultaneously. Decreasing values can be observed in PC1 of centroids, as incubation time increases, from the highest value at time 1 to negative values in this component at 30, 60 and 120 days.
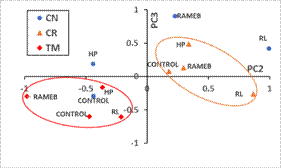
Otherwise, the effect of soil characteristics is mainly shown in PC2 (Figure 3, mean scores of each treatment plotted on the plane PC2 vs. PC3), where all treatments with soil CR (soil with the lowest OM) showed positive data in this PC2 and were clearly separated from treatments with soil TM (soil with the highest OM), that show negative values in all cases.
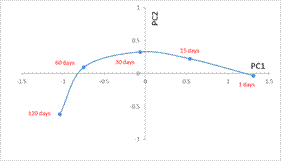
Figure 2 Evolution of scores corresponding to the whole set of data (centroid value at each incubation time for all the treatments simultaneously) plotted in the plane PC1 versus PC2.
Although OM is the main soil component responsible of increasing adsorption capacity of hydrophobic pollutants, our results (higher dissipation rates in TM) indicate that other soil properties should be considered, such as native degrader microorganisms. The points corresponding to treatments with soil CN (acidic pH, low clay, and medium OM content), have more disperse values and are not separated in PC2 from the other soils. The importance of these two factors (incubation time and soil type) in PAH dissipation agrees with results of Wu et al. (2014) that applied conjoint statistical analysis to PAHs amended contaminated soils. They also found these characteristics as the most significant factors in PAHs bioavailability.