Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Arquivos de Medicina
versão On-line ISSN 2183-2447
Arq Med vol.24 no.3 Porto jun. 2010
REVISÃO
Dementia and Cognitive Impairment: Clinical Diagnosis and Classification
Demência e Defeito Cognitivo: Diagnóstico Clínico e Classificação
Catarina Santos*,**, Nuno Lunet*
* Serviço de Higiene e Epidemiologia, Faculdade de Medicina da Universidade do Porto e Instituto de Saúde Pública da Universidade do Porto (ISPUP);
** Serviço de Neurologia, Centro Hospitalar de Coimbra
ABSTRACT
Dementia is an increasingly prevalent chronic condition, with a profound social impact. In the last few decades, emerging basic science concepts revealed the existence of various forms of dementia, with distinct neuropathological mechanisms. The two most representative subtypes of dementia in individuals over 65 years old are Alzheimer´s Disease and Vascular Dementia, with an increasingly recognized phenotypic overlap between these two entities. Mild Cognitive Impairment is an evolving operational concept, aiming to describe a transitional state between normal aging processes and dementia. Its relevance is nowadays undisputed, both at the clinical and scientific research level.
The diagnosis of both Dementia and Mild Cognitive Impairment are for the time being limited to clinical features and supportive auxiliary test results, which stresses the need for a thorough neuropsychological and neuropsychiatric assessment.
Key-words: dementia; mild cognitive impairment; diagnosis; classification
RESUMO
A demência é uma entidade crónica cada vez mais prevalente e que apresenta um importante impacto social. As descobertas recentes na área da ciência básica permitiram revelar a existência de vários tipos de demência, com mecanismos neuropatológicos distintos. As duas formas de demência mais frequentes em indivíduos com mais de 65 anos são a Doença de Alzheimer e a Demência Vascular, sendo a sobreposição fenotípica destas entidades cada vez mais reconhecida. O defeito cognitivo ligeiro é um conceito em evolução, que procura descrever a transição entre o processo normal de envelhecimento e a demência. A sua relevância é hoje indiscutível, quer do ponto de vista clínico quer no contexto de actividades de investigação. O diagnóstico de demência e de defeito cognitivo ligeiro está essencialmente dependente de aspectos clínicos, consubstanciados por resultados de alguns exames auxiliares de diagnóstico. A abordagem neuropsicológica e neuropsiquiátrica deve por isso ser particularmente rigorosa.
Palavras-chave: demência; defeito cognitivo ligeiro; diagnóstico; classificação
Introduction
Dementia is a chronic, debilitating condition, characterized by the deterioration of cognitive functions in several domains, in the absence of impairment of consciousness and persisting for a period of at least 6 months (1). Memory impairment is a typical, early and prominent feature in dementia, especially Alzheimers disease (AD), followed by executive function deterioration, apraxia, aphasia and/ or agnosia (1). Dementia is increasingly recognized as a manifestation of a degenerative process and not a disease itself. The cognitive and behavioural symptoms constitute only the tip of the iceberg, and the underlying pathological processes, although yet to be fully understood, may involve different degenerative pathways.
The existence of several diagnostic criteria for dementia should be kept in mind when analyzing and interpreting the findings from epidemiological studies. This applies in particular to the two most representative forms of dementia: AD and vascular dementia (VaD), where existing diagnostic criteria have been demonstrated not to be interchangeable (2-4). Mild cognitive impairment is a recently defined construct, aiming to identify the transitional state between non-pathological cognitive changes associated with aging and the earliest clinical features of dementia (5). This distinction is however not without difficulties and the criteria used to define mild cognitive impairment have evolved throughout the years (6); it is nevertheless an increasingly used construct inepidemiological studies. From the clinical point of view, the concept of early recognition of a neurodegenerative conditions has a relevant practical interest, especially when disease modifying treatments are a hope in the near future.
Clinical diagnosis and classification
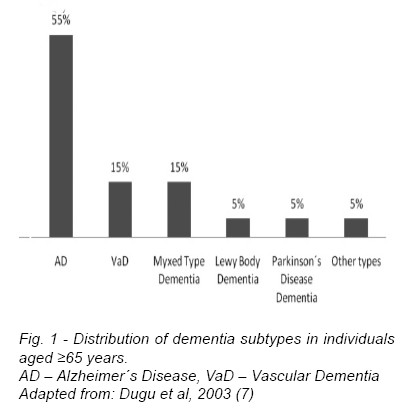
Several types of dementia have been described: AD, VaD, Lewy body, fronto-temporal dementia and dementia associated with Parkinson´s disease. Dementia may also be secondary to the human immunodeficiency virus (HIV-associated dementia) or other infectious agents, and this may assume particular importance in younger adults in specific world regions. Other rarer causes of dementia include Huntington´s disease, prion disease and head trauma dementia. AD is the most frequent subtype, corresponding to about 55% of all diagnoses in individuals aged above 65-years (7). Next in frequency is VaD, a frequent condition, especially in older people (8, 9), estimated to represent 15% of all cases (7), as illustrated in figure 1. Although AD can be identified with a considerable degree of accuracy, at present, there is no consensus on how to define mixed dementia in a clinical setting. Moreover, overlaping symptomathology, pathophysiology and comorbidity make the distinction between VaD and AD often difficult, and a differential diagnosis is further complicated by the fact that many patients have concomitant AD and cerebrovascular disease (10). In an elderly community-based autopsy study in the United Kingdom, AD was the primary pathological diagnosis in 59% of cases and VaD was considered present in 16% (11); nevertheless, when analyzing pathological findings regardless of the primary diagnosis, AD features were observed in 61% of cases and cerebrovascular pathology in 54%, highlighting the relevance of mixed dementia (11). The distinction between isolated AD, VaD, and mixed dementia coexisting in the same patient remains a controversial issue and one of the most difficult diagnostic challenges in this field (12).
Aiming the early detection of individuals at risk of dementia and seeking to delay the onset or progression of the disease, it has been an attempted to define earlier clinical stages of dementia. The consensual expression used to define this transitional state is Mild Cognitive Impairment (MCI), which is a conceptual rather than an operational definition, and the construct of this entity has evolved throughout the years. Initially, the term was used to reflect memory impairment with preserved non-memory cognitive performance and functional abilities (13). More recently, MCI was acknowledged to include impairment of cognitive domains other than memory (6), but it is presently unclear whether it should be strictly considered an early stage of a specific disease (AD being the paradigm), or rather, part of a broader syndrome. The criteria used to define MCI is a crucial methodological point to account for when comparing data as it may be responsible for heterogeneous findings across studies.
A recent study conducted in Portugal by Nunes et al (14) concluded that elderly individuals with cognitive complaints but normal performances on neuropsychological testing suffered a higher decline in total hippocampal volume during a 3.5-year follow-up. This suggests that cognitive complaints alone in elderly patients may preclude neurodegenerative changes and that pre-MCI could also be an interesting clinical and research concept to pursue (14).
Mild cognitive impairment
According to the original criteria dated from 1999, the definition of MCI relied heavily on memory as the main cognitive function affected, in the absence of functional dependence regarding daily life activities (5).
Two years later, Petersen et al (6) extended the definition and proposed the creation of four categories of MCI: amnestic versus non-amnestic and single versus multiple domains. This broader concept, however, does not specify the cognitive domains to be assessed when defining MCI, apart from memory, nor which neuropsychological instruments should be applied (6).
A diagnostic algorithm developed by Peterson et al (15) in 2004, requires an expression of concern regarding cognitive abilities from the patient or informant as the first step to define MCI. Clinicians should then assess whether the complainteffectively reflects cognitive impairment abnormal for age, along with normal daily functional abilities. Clinical history, mental status examination and neuropsychological testing are the main instruments available to perform this evaluation and demonstration of abnormal cognitive functioning for age, which may be quantified by reference to standard deviation from scores obtained by normal subjects within the same age range. The patient should be considered to have MCI if the previous assessment suggests that cognitive performance levels are neither normal nor compatible with dementia, and the individual is able to perform most daily activities.
This algorithm does not impose a defined mental status and neuropsychological approach and it is the clinician that ultimately defines the degree of functional disability of the individual (16). The European Alzheimers Disease Consortium/Alzheimers Disease Cooperative Study (EADC/ADCS) criteria were recently revised to allow for a mild decline in complex daily life activities when assessing MCI, extending the former concept of intact functional performance for the diagnosis of this condition (17).
In 2003, a group of clinicians and epidemiologists proposed a set of new working criteria to define MCI (18), in an attempt to provide clearer guidelines for clinical and research purposes. These criteria require a stepwise assessment of three diagnostic features:
1) Definition of not normal, but also not demented, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) or the International Classification ofMental and Behavioral Disorders (ICD-10) for a dementia syndrome;
2) Cognitive decline indicated by subject and/or informant report and objective cognitive tests;
3) Preserved basic activities of daily living with some minimal impairment in complex instrumental functions.
The need for subjective memory complaints may impair the sensitivity of criteria because patients with borderline dementia may have impaired awareness or deny cognitive problems, leaving the informants as the most reliable source of information. The 10/66 Dementia Research Group study has recently reported that in less developed countries the informants were less likely o report cognitive decline and social impairment (19), reflecting the social and cultural determinants of acknowledgement of this condition. The diversity of neuropsychological instruments used to evaluate cognitive performance and the different thresholds considered to define MCI also contribute to the arbitrary nature of this diagnosis. This was demonstrated by Larrieu et al (20) and Ritchie et al (17) in two separate longitudinal studies, in which the variability in defining MCI was partly attributed to differences in neuropsychological tests and cutoff scores used.
The lack of an operational definition of MCI adapted to the general population, as well as the fact that sample assessment at a single point in time may reflect a cohort effect are importantconcernswhen considering estimates of incidence and prevalence of MCI (18).
The difficulties in defining and diagnosing this entity are thus considerable, given the insidious manifestations, gradual progression of degenerative conditions and cultural issues. Nevertheless, MCI is a construct increasingly used in clinical practice and research, and is currently being considered for inclusion in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (21).
Alzheimer´s Disease
Brain imaging techniques have shown that AD patients have characteristic anatomical features distinguishing them from normal controls, namely atrophy of the temporal cortices, including the amygdala, hippocampus, and inferior temporal lobes, as well as of the anterior cingulate cortex (22).
Regardless of the recent advances in the knowledge of AD pathology, the clinical phenotype of this condition is largely non-specific and the definite diagnosis is ultimately neuropathological. Several criteria have been proposed, essentially based on the density of plaques and/or tangles, namely by the National Institute of Aging (23), the Consortium to Establish a Registry for Alzheimer´s Disease (CERAD) (24) and the Reagan Institute (25).
Despite the intense research on biological markers and neuroimaging techniques as potential aiding diagnostic tools, the recognition of AD in everyday practice and epidemiological surveys still relies essentially on clinical criteria (26).
Two sets of criteria have been developed for the clinical diagnosis of AD: the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), and the consensus criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer´s Disease and Related Disorders Association (NINCDS-ADRDA). In addition to these, the International Statistical Classification of Diseases and Related Health Problems (10th revision) also proposes a definition for Alzheimer´s disease.
The DSM-IV criteria are less extensive than those defined by NINCDS-ADRDA. DSM-IV requires only deficits in two or more cognitive domains with an impact on social and occupational performance, a progressive course and after exclusion of other potential causes, namely other systemic or psychiatric illnesses and delirium (27). The
NINCDS-ADRDA defines a more comprehensive set of criteria, proposing three categories of diagnoses: 1) definitive, when characteristic features are obtained from a neuropathological examination, 2) probable, when other likely causes of dementia are excluded and auxiliary diagnostic tests support the diagnosis, 3) possible, when other causes of dementia cannot be thoroughly excluded or atypical findings explained. According to this classification, the probable diagnosis of AD relies on the evidence of deficits in two or more areas of cognition, confirmed by neuropsychological evaluations (28). In parallel, it requires the exclusion of other causes of cognitive impairment, accounting for features that make the diagnosis of probableAD unlikely, such as suddenonset, focal neurological findings, seizures or gait disturbances early in the course of the illness (28).
For the diagnosis of AD, the ICD-10 requires a decline in memory and other cognitive abilities, characterized by deterioration in judgment and thinking, such as planning and organizing, and in the general processing of information, after the exclusion of other possible causes for cognitive deterioration such as alcohol, drugs or systemic disorders (29). This classification defines 4 categories for AD: early onset – before 65 years; late onset – after 65 years; atypical or mixed type; unspecified (29).
The NINCDS-ADRDA and the DSM-IV criteria are, however, the prevailing diagnostic standards in AD research (30), but both are likely to exclude broad populations of patients in very early stages of the disease, currently being labeled as having MCI (4). Despite the recent proposals for criteria incorporating data concerning cerebrospinal fluid analysis of amyloid beta or tau proteins as well as other biological markers (4), validation studies are still needed (4), and the DSM-IV and NINCDS-ADRDA criteria remain the mainstay of AD possible and probable diagnosis.
Vascular Dementia
The term vascular cognitive impairment refers to all clinical phenotypes where cognitive impairment is attributable to cerebrovascular disease, encompassing different forms of pathological findings, disease progression and clinical phenotypes. Vascular dementia thus represents a subset of this broad group.
The definition of the cognitive syndrome and the establishment of its vascular cause are critical elements in the concept and diagnosis of vascular dementia (VaD),for which there are four main sets of criteria available: DSM-IV, ICD-10, National Institute of Neurological Disorders and Stroke and Association Internationale pour la Rechérche et l`Enseignement en Neurosciences(NINDS-AIREN) and Alzheimer´s Disease Diagnostic and Treatment Centers (ADDTC) (31).
To define VaD, the DSM-IV criteria require the same general symptoms as for AD, but clinical or laboratory evidence is needed for a vascular cause for dementia (27). The latter requirements, however, are difficult to materialize because this set of criteria does not specify which brain imaging findings or focal neurological signs and symptoms should be valued.
The ICD-10 criteria define vascular dementia as the presence of all the general criteria for dementia and evidence of specific focal brain damage signs elicited in the neurological examination (29). Information from history or complementary tests of relevant cerebrovascular disease provides additional evidence of causal relationship.
The NINDS-AIREN criteria have been the most widely adopted in pharmacologic treatment trials and epidemiological studies (32). This is considered the most conservative classification since it requires imaging findings of vascular brain injury as well as focal signs on neurologic examination to define VaD (2). The ADDTC criteria also rely on radiological findings (i.e. evidence of two or more strokes outside cerebellum), but do not require focal neurological signs, and are therefore considered quite liberal (2).
Pohjasvaara et al (2) evaluated the use of different clinical definitions of VaD in case finding, in a series of 107 poststroke patients fulfilling the Diagnostic and Statistical Manual of Mental Disorders, Thirth Edition (DSM-III) criteria for dementia. These authors reported that defining VaD according to the different existing criteria, when compared to a DSM-III diagnosis, originated distinct frequency estimates. DSM-III and ICD-10 criteria had a concordance of 100%; the DSM-IV criteria were the most liberal and NINDS-AIREN the most restrictive classifications (2). Gold at al (3) described a sensitivity of 50% for the DSM-IV criteria contrasting with 20% for probable VaD according to the NINDS-AIREN, in when compared to the gold standard post-mortem neuropathological classification; the specificity for each set was 84% and 93%, respectively. The ICD-10 criteria showed diagnostic accuracy performance similar to the observed for the NINDS-AIREN (sensitivity, 20%; specificity, 94%). The existence of several non-interchangeable criteria for the diagnosis of vascular dementia raises important concerns when estimating the burden of disease in different settings, interpreting etiological research, and even making individual clinical decisions, stressing the need for standardization.
Neuropsychological evaluation
Neuropsychological assessment enables the characterization and quantification of the effects of brain damage on intellectual, motor or emotional functions. It is therefore an essential step in the diagnosis of dementia and detection of cognitive impairment, contributing to the identification of specific cognitive domains affected, and establishing a differential diagnosis with other important neuropsychiatric syndromes (33). These instruments are also important to monitor the progression of disease and symptomatic treatment efficacy (34).
There are several individual tests available to evaluate particular cognitive domains or behaviors (33), but the existing diagnostic criteria for dementia and MCI do not impose the use of specific tools for cognitive assessment.
The choice of tests or batteries from the large number of existing neuropsychological instruments is ultimately a decision of the clinicians or the researchers, largely governed by the available time and objectives to be accomplished with the evaluation. Therefore the option for different neuropsychological assessment tools is not necessarily homogeneous across the existing criteria, or within the same set of criteria used by different clinicians or researchers.
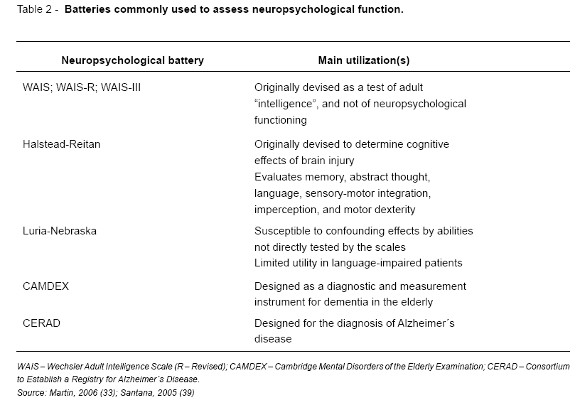
Tables 1 and 2 summarize the most widely used neuropsychological tests and batteries (33, 35-37).

Tests for screening of cognitive function are useful instruments for a mental status evaluation when the time available for the assessment is limited.
Dysfunction, which is a major limitation in the evaluation of cognition. In addition, it suffers from a strong ceiling effect and it is not suitable to identify slight declines when the levels of cognition are high (41).
The Clock-Drawing test is mainly used to assess visual-constructive skills, although it also provides valuable information on attention, episodic and semantic memory as well as executive and spatial capacities (42). There are several scoring scales for the Clock-Drawing test, assessing different cognitive components in distinct ways. There is presently a growing interest in the potential of this test as a screening bedside tool for cognitive impairment (43, 44); it is an easy and quick test to apply, with the objective of detecting moderate to severe cognitive impairment, although diagnostic validity is dependent on the Mini Mental State Examination (MMSE) is the best known and most extensively used of these tools, in both epidemiological studies and clinical practice. It includes questions on orientation, registration, attention and calculation, recall, language and visual construction (40). It is a valid test to define cognitive impairment, has high test-retest reliability, and the variation in the attained score in evaluations conducted in different moments is a good indicator of clinically meaningful cognitive decline (30). Although the average administration time is only 10 minutes (39), this test does not assess executive age and level of education (42). Other examples of screening tests are the Blessed Dementia Scale and the Alzheimer´s Disease Assessment Scale (ADAS) (39). The former briefly assesses aspects of daily life performance and changes in habits and personality, usually conveyed by the career. The ADAS was initially proposed as a practical instrument to monitor the efficacy of AD treatments, covering the cognitive areas most likely to be impaired in this form of dementia, with the notable exception of frontal dysfunction (37). It is commonly used to assess cognitive dysfunction (ADAS-Cog), as well as non-cognitive domains, in individuals with AD and other dementias.
Screening tests to be applied by telephone have been developed to be used in epidemiological surveys, of which the Telephone Interview for Cognitive Status (TICS) is the most notable example (45); some authors suggest that TICS provides a valid alternative to the MMSE and that scores obtained in both screening tests can be linked directly (46).
These screening tests cannot be used as an isolated instrument for the diagnosis of dementia, but they are often useful as an initial approach, preceding a more comprehensive and formal neuropsychological assessment.
Neuropsychological evaluation instruments in the Portuguese population
Factors such as language, reading ability, socioeconomic status, level of education and culture can strongly influence test performance. These instruments frequently have to be translated and also modified for a better adjustment to culture-specific references in different countries. The vocabulary, information and comprehension test components are the most often changed (33).
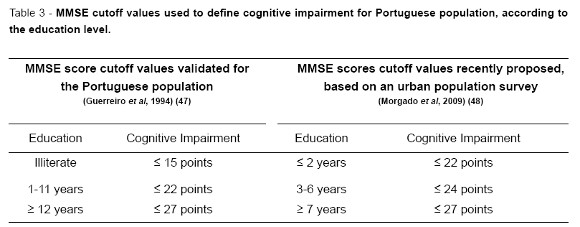
The most widely used test to screen for cognitive dysfunction, the MMSE, has been validated in the Portuguese population in 1994 by Guerreiro et al (47), establishing cutoff values according to formal education levels. A recent study conducted in the urban area of Lisbon by Morgado et al (48) proposed different cutoff values, reflecting cultural and social progresses over the past 20 years in Portugal. For the time being, the former criteria are still adopted when screening for cognitive impairment using the MMSE.
The ADAS-Cog screening test has a mean duration of administration of 20-30 minutes and is translated and validated in the Portuguese population, for different age groups and education levels (49).
The Bateria de Lisboa para Avaliação de Demência (BLAD) is the only comprehensive neuropsychological evaluation instrument designed and validated in the Portuguese population (50). It is more time-consuming than the ADAS, with a mean administration time of 90 minutes and requires specific training of the interviewers (39). The BLAD is composed of a number of tests that enable the characterization of levels of functioning localized in specific cortical areas and is useful in detecting executive dysfunction, which is frequently impaired in AD and other dementias (39). It is also suitable for the evaluation of populations with heterogeneous educational levels and constitutes the most widely recommended comprehensive instrument to corroborate the diagnosis of dementia in Portugal (39).
It must be stressed that cognitive assessment should always be complemented by an evaluation of depressive symptoms and other neuropsychiatric manifestations. It is also essential to assess the ability to perform daily life activities, as this is one of the criteria necessary to define dementia. The former objective can be accomplished by using the Neuropsychiatric Inventory, the Cornell Scale for Depression in Dementia and the Geriatric Depression Scale. The Instrumental Activities of Daily Living Scale and the Disability Assessment for Dementia Scale (38) are two of the most commonly used scales to characterize everyday functional performance. All of the referred scales and instruments are translated into Portuguese and available for clinical and research purposes (49).
Conclusion
The existing clinical criteria for AD and VaD are not interchangeable, which can give origin to discrepancies among study results. The understanding of the accuracy of each criteria set is crucial to both clinicians and researchers. A systematic, stepwise and multidisciplinary approach to the individual with cognitive complaints is fundamental to assure diagnostic accuracy, and the same principle may apply to epidemiological research.
References
1 -Fauman M. Delirium, Demência e Outras Perturbações. In: Fauman M, editor. Guia de Estudo para o DSM-IV TR. Lisboa: Climepsi Editores; 2002. [ Links ]
2 -Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et lEnseignement en Neurosciences. Stroke 2000;31:2952-7. [ Links ]
3 -Gold G, Bouras C, Canuto A, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry 2002;159:82-7. [ Links ]
4 -Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimers disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734-46. [ Links ]
5 -Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, KokmenE. Mild cognitive impairment: clinicalcharacterization and outcome. Arch Neurol 1999;56:303-8. [ Links ]
6 -Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985-92. [ Links ]
7 -Dugu M, Neugroschl J, Sewell M, Marin D. Review of dementia. Mt Sinai J Med 2003;70:45-53. [ Links ]
8 -Gay BE, Taylor KI, Hohl U, Tolnay M, Staehelin HB. The validity of clinical diagnoses of dementia in a group of consecutively autopsied memory clinic patients. J Nutr Health Aging 2008;12:132-7. [ Links ]
9 -Brunnstrom H, Englund E. Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psychiatry 2009;17:664-70. [ Links ]
10 -Roman G. Diagnosis of vascular dementia and Alzheimers disease. Int J Clin Pract Suppl 2001(120):9-13. [ Links ]
11 -Pathological correlates of late-onset dementia in a multi-centre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001;357:169-75. [ Links ]
12 -Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc 2002 ;50:1431-8. [ Links ]
13 -Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, et al. Apolipoprotein E status as a predictor of the development of Alzheimers disease in memory-impaired individuals. JAMA 1995;273:1274-8. [ Links ]
14 -Nunes T, Fragata I, Ribeiro F, et al. The outcome of elderly patients with cognitive complaints but normal neuropsychological tests. J Alzheimers Dis 2010;19:137-45. [ Links ]
15 -Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183-94. [ Links ]
16 -Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol 2004;61:59-66. [ Links ]
17 -Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001;56:37-42. [ Links ]
18 -Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240-6. [ Links ]
19 -Llibre Rodriguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 2008;372:464-74. [ Links ]
20 -Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002;59:1594-9. [ Links ]
21 -Petersen RC, OBrien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006;19:147-54. [ Links ]
22 -Poulin P, Zakzanis KK. In vivo neuroanatomy of Alzheimers disease: evidence from structural and functional brain imaging. Brain Cogn 2002;49:220-5. [ Links ]
23 -Khachaturian ZS. Diagnosis of Alzheimers disease. Arch Neurol 1985;42:1097-105. [ Links ]
24 -Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimers Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimers disease. Neurology 1991;41:479-86. [ Links ]
25 -Consensus recommendations for the postmortem diagnosis ofAlzheimers disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimers Disease. Neurobiol Aging 1997;18(4 suppl):S1-2. [ Links ]
26 -Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009;302:385-93. [ Links ]
27 -Association Psychiatric Association Diagnostic and statistical manual of mental disorders, DSM-IV. 4th ed. Washington DC: American Psychiatric Association;1994. [ Links ]
28 -McKhann G DD, Folstein M. Clinical diagnosis of Alzheimer´s disease: report of the NINDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer´s Disease. Neurology 1984;34:939-44. [ Links ]
29 -WorldHealthOrganization. Diagnostic Criteriafor Research [Internet].1993; Available from: http://www.who.int/clas-sifications/icd/en/GRNBOOK.pdf. [ Links ]
30 -Farlow M. Alzheimer´s Disease. In: Miller A, editor. Continuum Lifelong Learning in Neurology - Dementia. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Links ]
31 -Chui H, Nielsen-Brown N. Vascular Cognitive Impairment. In: Miller A, editor. Continuum Lifelong Learning in Neurology - Dementia. Philadelphia: Lippincott Williams & Wilkins; 2007. [ Links ]
32 -Wiederkehr S, Simard M, Fortin C, van Reekum R. Validity of the clinical diagnostic criteria for vascular dementia: a critical review. Part II. J Neuropsychiatry Clin Neurosci 2008;20:162-77. [ Links ]
33 -Martin G. Neuropsychological assessment. In: Martin G, editor. Human Neuropsychology. 2nd ed. Harlow: Pearson Prentice Hall; 2006. [ Links ]
34 -Hodges J, editor. Cognitive Assessment for Clinicians. 2nd ed. New York: Oxford University Press; 2007. [ Links ]
35 -Roth M, Tym E, Mountjoy CQ, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698-709. [ Links ]
36 -Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimers disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimers Disease. Arch Neurol 1992;49:448-52. [ Links ]
37 -Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimers disease. Am J Psychiatry 1984;141:1356-64. [ Links ]
38 -Guerreiro M. Avaliação Neuropsicológica das Demências Degenerativas. In: Castro-Caldas A, de Mendonça A, editors. A Doença de Alzheimer e outras demências em Portugal.1st ed. Lisboa: Lidel; 2005. [ Links ]
39 -Santana I. Avaliação Neuropsicológica. In: Santana I, Cunha L, editors. Demência(s): Manual para Médicos. 1st ed. Coimbra: Faculdade de Medicina, Universidade Coimbra; 2005. [ Links ]
40 -Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922-35. [ Links ]
41 -Commenges D, Gagnon M, Letenneur L, Dartigues JF, Barberger-Gateau P, Salamon R. Statistical description of the Mini-Mental State Examination for French elderly community residents. Paquid Study Group. J Nerv Ment Dis 1992;180:28-32. [ Links ]
42 -Silva D, de Mendonça A, Guerreiro M. The Clock-Drawing Test: historical notes followed by a few examples. Sinapse 2009;9:52-7. [ Links ]
43 -CachoJ, Garcia-GarciaR, Arcaya J, Vicente JL, LantadaN. [A proposal for application and scoring of the Clock Drawing Test in Alzheimers disease]. Rev Neurol 1999;28:648-55. [ Links ]
44 -Watson YI, Arfken CL, Birge SJ. Clock completion: an objective screening test for dementia. J Am Geriatr Soc 1993 Nov;41(11):1235-40. [ Links ]
45 -Wolfson C, Kirkland SA, Raina PS,et al. Telephone- administered cognitive tests as tools for the identification of eligible study participants for population-based research in aging. Can J Aging 2009;28:251-9. [ Links ]
46 -Fong TG, Fearing MA, Jones RN, et al. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement 2009;5:492-7. [ Links ]
47 -Guerreiro M, Silva A, Botelho M, Leitão O, Castro-Caldas A, Garcia C. Adaptação à população portuguesa da tradução do Mini Mental State Examination (MMSE). Revista Portuguesa de Neurologia 1994;1. [ Links ]
48 -Morgado J, Rocha C, Maruta C, Guerreiro M, Pavão Martins I. Novos Valores Normativos do Mini-Mental State Examination. Sinapse 2009;9:10-6. [ Links ]
49 -Guerreiro M, Fonseca S, Barreto J, et al. Escala de Avaliação da Doença de Alzheimer. Escalas e Testes na Demência 2003:27-32. [ Links ]
50 -Garcia C. A Doença de Alzheimer: Problemas de Diagnóstico Clínico [PhD Thesis]. Faculdade de Medicina da Universidade de Lisboa; 1984. [ Links ]
Dr.ª Catarina Santos
Serviço de Higiene e Epidemiologia
Faculdade de Medicina da Universidade do Porto Alameda Prof. Hernâni Monteiro 4200-319 Porto. E-mail: catarina-santos@portugalmail.pt














