INTRODUCTION
The link between asthma and obesity is well-established, with numerous studies highlighting obesity as a risk factor for asthma development and exacerbation. Obese individuals are not only more likely to develop asthma but also experience more severe symptoms, reduced treatment efficacy, and frequent exacerbations, leading to a diminished quality of life. Several overlapping mechanisms drive this multifactorial relationship, including mechanical, metabolic, and immunological factors 1,2.
Mechanically, obesity increases thoracic and airway compression, leading to reduced lung compliance and impaired lung function, including reductions in expiratory flow and lung volumes and airway hyperresponsiveness. Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) tend to be mildly reduced in obese individuals, resulting in a normal or close to normal FEV1/FVC ratio. Maximum expiratory flow 25-75% of FVC can be markedly diminished, reflecting small airway obstruction 3.
Systemically, obesity contributes to a chronic low-grade inflammatory state characterized by elevated pro-inflammatory mediators such as leptin, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) 4). These mediators worsen asthma by increasing airway inflammation and reducing the efficacy of inhaled corticosteroids (ICS), a primary treatment for asthma. Additionally, metabolic abnormalities, oxidative stress, mitochondrial dysfunction, and immune dysfunction are also encountered in obese individuals 5. Comorbidities associated with obesity, such as gastroesophageal reflux disease, obstructive sleep apnea, and anxiety/depression, further complicate asthma management 1,2.
In obese patients, weight loss of as little as 5-10% has been shown to improve asthma control, enhance lung function, and reduce the need for asthma medications6.
Bariatric surgery, the most effective intervention for significant and sustained weight loss, has also been shown to improve asthma control, making it a viable option when less invasive approaches prove unsuccessful 7,8. Studies on morbidly obese patients [Body Mass Index (BMI) > 40 kg/m2] consistently report improvements in asthma symptoms, medication use, exacerbation rates, and quality of life following surgery 8,9.
Recent studies have also explored bariatric surgery in patients with class I obesity (BMI between 30-39.9 kg/ m²). While these studies are scarce, evidence suggests that patients in this BMI category may experience significant improvements in asthma control and related comorbidities 1,10. Moreover, airway hyperresponsiveness appears to improve to a greater extent in obese asthmatics with normal serum IgE 10, indicating that specific asthma phenotypes may benefit more from bariatric surgery than others. This late-onset, non-atopic phenotype, often seen in post-menopausal women and characterized by neutrophilic airway inflammation, suggests that surgery may provide therapeutic benefits beyond weight reduction alone 11.
In Portugal, national guidelines (General Direction of Health, guideline n.º 028/2012) currently restrict bariatric surgery to individuals with BMI > 40 kg/m2 or BMI > 35 kg/m2 with specific comorbidities 12. However, BMI alone may be insufficient for adiposity, metabolic disease, and cardiovascular risk. Recent evidence suggests that bariatric surgery should be considered for patients with a BMI > 30 kg/m2 individually, following a comprehensive clinical assessment of their overall health. This approach aligns with recommendations from international organizations, including the American Diabetes Association, which advocates for bariatric surgery in individuals with a BMI > 30 kg/m² who have uncontrolled comorbidities such as type 2 diabetes, especially when glycemic control remains inadequate despite lifestyle and medical interventions 13-15. Given the overlap between metabolic syndrome, inflammation, and asthma, it is plausible that bariatric surgery could also benefit obese asthmatic patients within this BMI range. However, research in this specific population is limited.
Addressing this gap is crucial, as uncontrolled asthma in obese patients not only reduces their quality of life but also imposes a significant economic burden on healthcare systems. This prospective observational study aims to investigate the effects of bariatric surgery on asthma control, quality of life, and lung function in patients with a BMI between 30-39.9 kg/m², providing evidence on whether these patients experience benefits similar to those observed in the morbidly obese population.
OBJECTIVES
To investigate the impact of bariatric surgery on asthma control in obese patients.
Primary Outcome:
• Asthma Control Test (ACT) score/Control of Allergic Rhinitis and Asthma Test (CARAT) score. Secondary outcomes:
• Mini Asthma Quality of Life Questionnaire (MiniAQLQ) score;
• Clinically significant exacerbation;
• Emergency room (ER)/hospital visits;
• Oral Corticosteroids (OCS) use;
• Rescue inhaled medication use;
• Lung function, namely forced expiratory volume in the first second (FEV1), forced mid-expiratory flow between 25% and 75% of forced vital capacity (FEF25-75), bronchial reversibility;
• Fractional exhaled nitric oxide (FeNO).
METHODS AND ANALYSIS
Participants, interventions, and outcomes
This prospective observational study will include obese patients with difficult-to-treat or uncontrolled asthma. Adult asthma patients with BMI >30 kg/m2, who have been unable to lose weight through lifestyle changes and medical treatment, and who are being followed in the Allergy and Clinical Immunology Department at a University Hospital in Portugal, or those referred for bariatric surgery at the same academic hospital with a confirmed diagnosis of difficult-to-treat or uncontrolled asthma will be recruited.
Subject Inclusion Criteria
To be eligible for participation in this study, subjects must meet all the following criteria:
• Aged between 18 and 65 years;
• Have a confirmed asthma diagnosis through lung function tests;
• Suffer from difficult-to-treat or uncontrolled asthma, as defined by GINA guidelines;
• BMI > 30 kg/m2;
• Failure to achieve weight loss through non-surgical options after at least one year;
• Willingness to comply with all study procedures and availability for the duration of the study;
• Ability to understand the surgical procedure and commitment to the long-term follow-up program;
• Provide a signed and dated informed consente form.
Subject Exclusion Criteria
Potential subjects will be excluded from the study if they meet any of the following conditions:
• Obesity due to untreated endocrine disease;
• Unstable psychiatric conditions.
• Alcohol or drug abuse;
• Pregnancy.
Eligible patients will be recruited and invited to participate in the study.
Intervention
Patients enrolled in our study will undergo bariatric surgery, with the specific procedure selected based on individual characteristics, comorbidities, previous treatments, patient preferences, and the recommendations of the surgical team. Patients will be advised throughout the study to continue their prescribed asthma treatment.
Before surgery, ideally during the pre-operative consultation, asthma control and quality of life will be assessed using the ACT, CARAT, and AQLQ questionnaires. Additionally, spirometry will be performed, and a blood sample will be collected to measure eosinophil count and total serum IgE. These assessments will be repeated one year after surgery (Table 2). Post-bariatric clinical evaluations and testing will be scheduled on the same day as the patient’s general surgery follow-up appointment to promote participant retention. Our hospital also provides appointment reminders one day prior to the scheduled visit, and if patients miss their appointment, they will be contacted by phone to arrange a new one.
Outcomes Assessment
Patients will be asked to complete three questionnaires - ACT, CARAT, and MiniAQLQ - before surgery and again one year afterward. The scores will be used to assess each patient’s level of asthma control (Table 1) and quality of life.
Table 2 Schedule of events. All visits will be in-clinic.
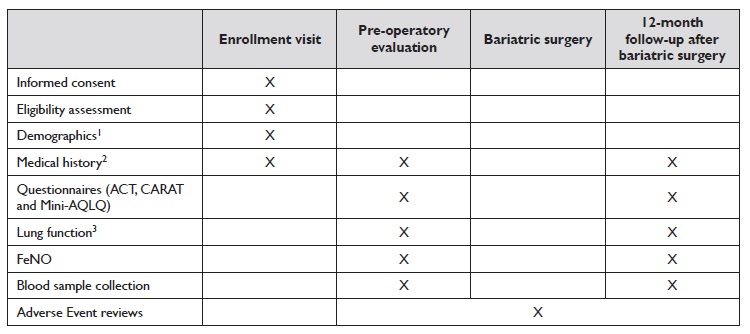
Abbreviations: ACT - asthma control test; CARAT - control of allergic rhinitis and asthma test; FeNO - fractional exhaled nitric oxide; MiniAQLQ - mini asthma quality of life questionnaire.
1 Demographics: Year of birth; height (meters [m]; without shoes); weight (kilograms [kg]); race; and sex.
2 Medical history includes asthma symptoms, as well as number of exacerbations, oral corticosteroids and medication step-up/ down.
3 Lung function includes forced expiratory volume in the first second, forced mid-expiratory flow between 25% and 75% of forced vital capacity, and bronchial reversibility.
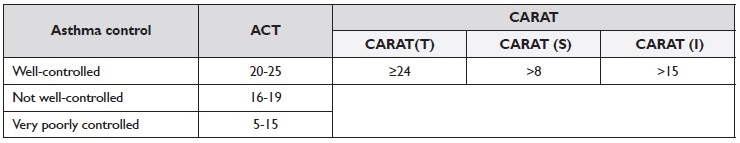
ACT is a self-reported questionnaire with five items that assess asthma symptoms, the use of rescue medications, and the impact of asthma on daily functioning. Each item is rated on a scale from one to five, and a total score is obtained from five (poor asthma control) to 25 (complete control of asthma) 16.
CARAT is a patient-completed questionnaire that evaluates the control of allergic rhinitis and asthma over the previous four weeks. It consists of ten questions divided into two sections: CARAT(S) for upper airway symptoms and CARAT(I) for lower airway symptoms.
One additional question addresses sleep disruption, and another concerns the use of additional medications. Each question is scored from 0-4, with the total score [CARAT(T)] ranging from zero to 30, where lower scores indicate worse control and higher scores indicate better control 17.
MiniAQLQ assesses functional problems (physical, emotional, social, and occupational) that patients have experienced in the past two weeks due to asthma. This 15-question survey is divided into four domains (symptoms, activity limitation, emotional function, and environmental stimuli) and takes approximately 3-4 minutes to complete. The overall score is the mean of all 15 responses, rated on a 7-point scale (7 = not impaired at all, 1 = severely impaired) 18.
A clinically significant asthma exacerbation is defined as a worsening of asthma that requires OCS use for at least three days, an emergency department visit, or hospitalization that requires systemic corticosteroids. 19.
Lung function will be evaluated using spirometry with reversibility testing, conducted by a trained professional in accordance with the recommendations of the European
Respiratory Society/American Thoracic Society (ERS/ ATS) using a JAEGER spirometer 20.
The results obtained will help determine the efficacy of bariatric surgery in these patients.
The approximate time to complete study enrollment is two years.
The expected duration of each participant’s involvement, aside from the bariatric surgery itself, is approximately two hours, requiring two visits to the Allergy and Clinical Immunology Department.
Power calculations assumed that the ACT score would improve by an average of 3 points (with a standard deviation of 3) 12 months post-surgery 21. With a Type I error rate of 0.05, a sample size of 18 subjects would provide over 85% power to detect a significant difference.
Anticipating a 10% loss to follow-up during the 12-month study period, we plan to recruit 20 asthmatic patients.
Recruitment
The protocol will be disseminated within the Allergy and Clinical Immunology Department and to the bariatric surgical team at the same University Hospital. Potential participants are expected to be promptly evaluated following referral.
Statistical considerations
Statistical analysis will be performed using IBM SPSS Statistics 29®.
Descriptive statistics will be provided for the number of participants, sex, age, blood sample parameters (including blood eosinophils and total IgE), scores from ACT, CARAT, and MiniAQLQ questionnaires, number of clinically significant exacerbations and ER/Hospital visits, OCS use, rescue inhaled medication use, FEV1, FEF25-75 and the presence of bronchial reversibility.
Absolute and relative frequencies will be reported for categorical variables. The mean and standard deviation for quantitative variables will be presented for normally distributed data, while the median and interquartile range will be used for non-normally distributed data. Both parametric and non-parametric tests will be applied, depending on the data distribution. Statistical significance will be set at p-values < 0.05.
EXPECTED RESULTS AND POTENTIAL LIMITATIONS
At an individual level, participants in bariatric surgery often experience substantial and sustained weight loss, which can lead to improved asthma control, reduced medication use, fewer exacerbations, enhanced quality of life, and lowered cardiovascular risk. Each case will be carefully evaluated to ensure the potential benefits outweigh the risks. Patients will be followed by a multidisciplinary team experienced in bariatric surgery to minimize complications.
This study aims to understand better the impact of bariatric surgery on obese asthmatic patients, taking into account asthma phenotype and baseline BMI. Based on previous research involving patients with morbid obesity and/or diabetes, we expect improved asthma control following surgery.
However, the study’s limited sample size and stringent inclusion/exclusion criteria may limit the generalizability of the findings. Additionally, the absence of a control group could weaken the ability to draw definitive conclusions regarding the link between the intervention and the outcomes. Other potential biases also exist. Participants with higher BMI may be more motivated to enroll, leading to non-response bias. Furthermore, performance bias might occur due to differences in surgical techniques or postoperative care associated with different bariatric surgery types.
For future research, we recommend a randomized control trial comparing outcomes between obese asthmatic patients who undergo bariatric surgery and a matched control group receiving standard weight management interventions, such as lifestyle modifications and pharmacological treatments. This design would help minimize selection bias and provide more precise insights into the specific benefits of bariatric surgery.
ETHICS AND DISSEMINATION
The study follows Good Clinical Practice according to the Declaration of Helsinki. Ethical approval was granted by the University Hospital (nº 243/21). In the case of protocol amendments, relevant parties will be informed.
All participants will provide informed consent. All patients will receive an information leaflet explaining the study’s rationale and objectives and the potential risks, expected benefits, patients’ rights, and investigator contact. The surgical team will explain the risks associated with the chosen procedure.
During and after the study, patients will maintain regular follow-ups with both the Allergy and Clinical Immunology Department and the Integrated Responsibility Unit - Obesity, a multidisciplinary team specialized in following these patients after surgery. Patients will be referred to specialists for any medical conditions requiring specialized care outside of these areas if necessary.
Participants’ personal data will be anonymized using a pseudonymization process to ensure confidentiality.
Only the principal investigator can access the key to re-identify the data if necessary. All personnel involved in data collection and management have training in Good Clinical Practice and data handling procedures to ensure data quality and consistency. Data will be regularly reviewed by the research team to identify and address any missing or inconsistent entries.
The study results are expected to be published in a scientific journal. Upon completion of the study, all participants will receive information on the results if desired.