Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.1 Lisboa jan. 2012
The use of statins in patients with chronic kidney disease not in dialysis. A scientific review
António Vaz Carneiro, MD, PhD, FACP
Center for Evidence-Based Medicine.University of Lisbon School of Medicine. Portugal. Cochrane Coordinating Center, Portugal.
ABSTRACT
Chronic kidney disease is a major risk factor for the incidence and severity of coronary artery disease. Patients with CKD present accelerated atherosclerosis and are prone to serious heart disease, including heart failure, before they ever reach dialysis. They have a worse cardiovascular (CV) prognosis then other patients after acute myocardial infarction (AMI), and after revascularisation.
The main aim of this review article is the presentation and discussion of the best available evidence on the use of statins in patients with hyperlipidaemia and CKD not on dialysis. This paper is not based on a systematic review of the best clinical evidence on the subject of statins and CKD. It is a scientific review based on recent studies (randomised controlled trials, systematic reviews and observational studies) on risk modulation with lipid-lowering drugs in CKD. The evidence on which this paper is based was identified by searching the best available secondary sources as well as primary databases if needed.
There are a series of statements that can be made on the effects of statins in patients with CKD not on dialysis. Firstly, the combination ezetimibe/simvastatin reduces AMI, non-haemorrhagic stroke and revascularisation in these patients, and a physician needs to treat less than 50 patients over four years to avoid a CV event. Secondly, the combination ezetimibe/simvastatin reduces LDL levels more than simvastatin alone. Thirdly, treatment with atorvastatin plus ACE inhibitors or ARBs may reduce proteinuria and the rate of progression of kidney disease, proteinuria and hypercholesterolaemia. Fourthly, pravastatin was associated with slower renal function decline than placebo in patients with moderate reduced GFR and proteinuria. Fifthly, simvastatin has similar effects on total cholesterol, LDL and triglyceride in CKD patients as it has in patients with normal kidney function. Sixthly, statin use is associated with reduction in albuminuria or proteinuria. Seventhly, statin therapy with rosuvastatin reduced first cardiovascular events and all-cause mortality among men and women with low LDL, elevated CRP and moderate CKD. Finally, pravastatin may not be superior to usual care in preventing end-stage renal disease and addition of atorvastatin to angiotensin-converting enzyme inhibitor or angiotensin receptor blocker may slow progression of renal disease.
Key-Words:Cardiac prognosis; chronic kidney disease; chronic renal disease; coronary artery disease; statins.
INTRODUCTION
Chronic kidney disease (CKD), also called chronic renal disease, is a major risk factor for the incidence and severity of coronary artery disease (CAD )1. Cardiovascular (coronary) disease is the most frequent cause of death among CKD patients and less than 2% of CKD patients eventually require renal replacement therapy2.
Patients with CKD present accelerated atherosclerosis and are prone to serious heart disease (including heart failure) before they ever reach dialysis. They have a worse cardiovascular (CV) prognosis then other patients after acute myocardial infarction (AMI)3, and after revascularisation4.
Observational studies have shown that lowered glomerular filtration rate (GFR) and proteinuria are independent and major risk factors for CV disease5. In a recently published collaborative-analysis6, standardised data for all-cause and cardiovascular mortality were pooled to estimate hazard ratios (HRs) for all-cause and cardiovascular mortality associated with estimated glomerular filtration rate (eGFR) and albuminuria, adjusted for potential confounders. The sample included 105,872 participants (730,577 person-years) from 14 studies with urine albuminto-creatinine ratio (ACR) measurements showing that the risk of mortality was increased at lower eGFRs. That is, HRs were 1.18 (95% CI 1.05-1.32) for eGFR=60 mL/min/1.73 m2, 1.57 (1.39-1.78) for 45 mL/min/1.73 m2, and 3.14 (2.39-4.13) for 15 mL/min/1.73 m2.
The sample also included 1,128,310 participants (4,732,110 person-years) from seven studies with urine protein dipstick measurements. The estimated glomerular filtration rate and ACR were associated also with risk of cardiovascular mortality, and in studies with dipstick measurements, proteinuria was an independent prognostic factor.
Treatment of CV risk factors in CKD patients – i.e.control of arterial hypertension, smoking cessation, weight loss and control of hyperglycaemia in diabetics and modulation of serum cholesterol – is of paramount prognostic importance, since they tend to significantly lower mortality (inconsistently) and morbidity in patients with renal failure7.
Prognosis of CKD patients with cardiac disease is poor. A recent retrospective analysis of 259 patients with CKD found that most of them had several cardiac risk factors8: hypertension in 87%, diabetes in 35%, cardiovascular disease in 40% and peripheral vascular disease in 14%. Forty-seven per cent of these patients were hospitalised after a median follow-up of 11.4 months. Cardiovascular disease (excluding congestive heart failure) or hypertension were the primary diagnoses in approximately 25% of these hospitalisations.
Until recently there was scanty evidence of the benefit of lipid-lowering therapy in patients with CKD, since CKD is generally an exclusion criterion for the trials modulating CV risk factors9. The only three published clinical trials in patients on dialysis (or post-kidney transplantation) using statins – atorvastatin10, rosuvastatin11and fluvastatin12 – did not show a significant reduction in primary CV outcome, casting doubts on the benefit of this secondary preventive measure (since CKD is considered a CAD equivalent).
The same absence of benefit on mortality was seen on a recent-analysis7 which showed however that statin therapy significantly reduced lipid concentrations in patients with chronic kidney disease (all stages of disease). The results were total cholesterol (42 studies, 6,390 patients; WMD −42.28 mg/dl (1.10 mmol/l), 95% CI −47.25 to −37.32), and low density lipoprotein cholesterol (39 studies, 6,216 patients; −43.12 mg/dl (1.12 mmol/l), 95% CI −47.85 to −38.40) – and CV end points – proteinuria (g/24 hours) (6 trials, 311 patients; 95% CI −0.73 g/24 hour −0.95 to −0.52) – but no benefit on all-cause mortality (44 studies, 23,665patients; 0.92, 95% CI 0.82 to 1.03). In this study no role of statins in primary prevention was established.
So the question seems to be: what is the real benefit of lipid-lowering therapy with statins in patients with CKD not on dialysis?
In this paper we briefly review the most recent scientific evidence on the use of statins in patients with chronic kidney disease not in dialysis, and the effect of therapy on clinical outcomes.
METHODOLOGY
The main aim of this paper is the presentation and discussion of the best available evidence on the use of statins in patients with hyperlipidaemia and CKD not on dialysis. No specific subgroups were selected.
This paper is not based on a systematic review of the best clinical evidence on the subject of statins and CKD. It is a scientific review based on recent studies (randomised controlled trials, systematic reviews and observational studies) on risk modulation with lipid-lowering drugs in CKD.
The evidence on which this paper is based was identified by searching the following secondary sources: Clinical Evidence, Essential Evidence Plus, Evidence Based Medicine, ACP Journal Club, UpTo-Date, Cochrane Library, DynaMed, NICE, Evidence Based Practice, Guideline International Network, National Guideline Clearinghouse, Essential Evidence Plus, ACP's PIER and Scottish Intercollegiate Guidelines Network.
If the dates of the aforementioned sources were significantly outdated, Medline was searched also from the latest date to the present (December 2011).
LIPID ABNORMALITIES IN PATIENTS WITH CKD
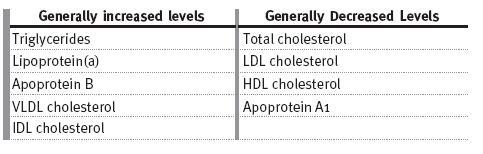
The most common lipid abnormality in CKD patients is hypertriglyceridaemia, due to defective removal from the circulation and associated secondary hyperparathyroidism. Table I shows the alterations in lipid profiles in CKD13. In CKD patients total cholesterol concentration can be high (20-30% of patients), normal or low. HDL is normally low14, and plasma levels of lipoprotein(a) are usually elevated15.
Table I
Changes in lipids in CKD
In a recent paper16, researchers examined whether malnutrition or inflammation (M-I) would modify the relationship between cholesterol levels and CVD events in African-Americans with hypertensive CKD and a GFR between 20 and 65 ml/min per 1.73 m2. 990 participants were stratified by the presence or absence of M-I (defined as body mass index<23 kg/m2 or C-reactive protein>10 mg/L at baseline). The primary composite outcome included cardiovascular death or first hospitalisation for coronary artery disease, stroke, or congestive heart failure, occurring during a median follow-up of 77 months.
In adjusted analyses, the CVD composite outcome exhibited a significantly stronger relationship with total cholesterol for participants without M-I than for participants with M-I at baseline (P<0.02). In the non-M-I group, the cholesterol-adjusted hazard ratio (HR) for CVD increased progressively across cholesterol levels: HR = 1.19 [95% CI; 0.77, 1.84] and 2.18 [1.43, 3.33] in participants with cholesterol 200 to 239 and≥240 mg/dl, respectively. In the M-I group, the corresponding HRs did not vary significantly by cholesterol level. The authors concluded that the presence of M-I modifies the risk relationship between cholesterol level and CVD in African-Americans with hypertensive CKD.
(3-Hydroxyl-3-methylglutaryl CoA reductase inhibitors) and other lipid-lowering drugs have been clearly demonstrated to diminish the morbidity and mortality of CV disease in the general population. Hence, there is hope that the use of these drugs may likewise help patients with CKD.
Also, it has been theorised that dyslipidaemia may contribute to the progression of CKD, so that lipid-lowering drugs may prove to slow CKD progression. The current medical literature provides insufficient data to guide the use of lipid-lowering drugs in the CKD population, in large part because CKD has been an exclusion criterion for most of the RCTs that have addressed this issue.
We selected clinical evidence on the effect of statins in patients with CKD and their benefit on a number of clinical outcomes.
TREATMENT OF CKD PATIENTS WITH STATINS
The effects of statin therapy on CKD patients were analysed in the ten studies below.
1. Bianchi S et al. trial, 200317
In a prospective, controlled, open-label randomized trial, 56 patients with nondiabetic kidney disease who were treated for one year with ACE inhibitors or ARB and other antihypertensives were randomized to atorvastatin (atorvastatin group) vs. no additional treatment (control group)17. After twelve months, urine protein excretion decreased in the atorvastatin group (from 2.2 to 1.2 g/24 hours, P <0.01) and the control group showed no significant changes (from 2 to 1.8 g/24 hours, P=NS). Creatinine clearance did not decrease significantly in the experimental group (from 51 to 48.8 mL/minute) but decreased in the control (from 50 to 44.2 mL/minute). These results suggests that treatment with atorvastatin plus ACE inhibitors or ARBs may reduce proteinuria and the decline in renal function in patients with CKD, proteinuria, and hypercholesterolaemia, and the benefits appear to occur in addition to those of treatment with ACE inhibitor and ARBs alone.
2. Post-hoc analysis of the CARE trial, 200318
Another published study18 was a posthoc subgroup analysis of a randomised double-blind placebo controlled trial – the CARE trial27 – to determine whether pravastatin reduced rates of loss of renal function in people with moderate CKD. In 690 patients (20.4% from 3,384 randomised patients) with eGFR <60 mL/minute/1.73 m2 (MDRD-GFR), no significant differences in rate of decline in eGFR comparing pravastatin vs. placebo was found (0.1 ml/min per 1.73 m2/yr slower; 95% CI-0.2 to 0.4; P = 0.49). Sub-subgroup analyses found that pravastatin was associated with slower renal function decline than placebo in patients with eGFR <50 mL/minute/1.73 m2 (difference 0.6 mL/minute/1.73 m2/year), eGFR <40 mL/minute/1.73 m2 (difference 2.5 mL/minute/1.73 m2/year) and proteinuria (P=0.006). This result was not verified in another subgroup analysis of the ALLHAT trial28.
3. PREVEND-IT trial, 200419
The Prevention of Renal and Vascular End-Stage Disease Intervention Trial (PREVEND IT)19, performed at a single Dutch centre, was a double-blind, randomised, placebo-controlled trial designed to assess whether lowering urinary albumin excretion would reduce cardiovascular events in low risk CKD (microalbuminuric subjects). The trial had a 2 × 2 factorial design involving fosinopril or placebo and pravastatin 40 mg or placebo. Patients (n=864, from a previous cohort) were 28 to 75 years of age (51±12 years; 65% of subjects male; 3.4% had a previous cardiovascular event) and were found to have microalbuminuria (15 to 300 mg/24 hours) on screening.
Patients were excluded when the creatinine clearance was 60% higher than the normal age-adjusted value.
The mean follow-up was 46 months, and the primary end point was cardiovascular mortality and hospitalization for cardiovascular morbidity. Patients were randomised to fosinopril 20 mg or matching placebo and to pravastatin 40 mg or matching placebo. Mean cholesterol level was 5.8±1.0 mmol/L, mean systolic/diastolic blood pressure 130±18/76±10 mm Hg, and median urinary albumin excretion 22.8 (15.8 to 41.3) mg/24 hours.
Although fosinopril produced a significant (26%, P<0.001) reduction in albuminuria in 45 subjects (5.2%) and was associated with a trend of decreasing cardiovascular events (HR 0.60; 95% CI 0.33 to 1.10; P<0.098), treatment with pravastatin did not result in a significant reduction in urinary albumin excretion or a reduction in cardiovascular events (HR 0.87; 95% CI 0.49 to 1.57; P<0.649). The authors concluded that in this study, treatment with pravastatin did not result in a significant reduction in urinary albumin excretion or cardiovascular events.
4. UK-HARP-I trial, 200520
Two studies were designed to establish the biochemical efficacy and to gain more information on the safety of cholesterol-lowering drugs in patients with CKD.
In the first UK Heart and Renal Protection study (UK-HARP-I)20, 448 patients with CKD were randomly assigned in a 2 × 2 factorial design to the administration of 20 mg of simvastatin daily versus matching placebo, and 100 mg of modified-release aspirin daily versus matching placebo. At the start, 242 patients were predialysis (creatinine level ≥ 1.7 mg/dL), 73 were receiving maintenance dialysis, and 133 had a functioning transplant. Among those with predialysis, renal failure or a functioning transplant at baseline, aspirin did not increase the number of patients who progressed to dialysis therapy (4% versus 3% patients; P=NS) or experienced a greater than 20% increase in creatinine level (34% versus 30%; P=NS).
After a follow-up of 12 months, allocation to 20 mg of simvastatin daily reduced non-fasting total cholesterol levels by 18% (simvastatin, 163 mg/dL versus placebo, 196 mg/dL, P<0.0001), LDL levels by 24% (89 mg/dL versus 114 mg/dL; P<0.0001), and triglyceride levels by 13% (166 mg/dL versus 186 mg/dL; P<0.01). There was no significant effect on highdensity lipoprotein cholesterol levels (2% increase; P=NS). The simvastatin group did not have a significantly increased risk for abnormal liver transaminases, elevated creatinine kinase, or clinical myopathy.The authors conclude that simvastatin 20 mg/d produced a sustained reduction of approximately 25% in LDL levels, with no evidence of toxicity, and aspirin, 100 mg/d, did not substantially increase the risk for a major bleeding episode.
5. UK-HARP-II trial, 200621
The second UK Heart and Renal Protection study (UK-HARP-II )21 was designed to assess the tolerability, safety and biochemical efficacy of ezetimibe (10 mg/d) in combination with simvastatin (20 mg/d). A sample of 203 patients (152 predialysis patients with creatinine levels >1.7 mg/dL, 18 patients on peritoneal dialysis therapy and 33 patients on haemodialysis therapy) were randomly assigned to the administration of simvastatin, 20 mg/d plus ezetimibe, 10 mg/d; or simvastatin, 20 mg daily plus placebo ezetimibe daily.
Six months later, patients taking simvastatin alone observed a 31-mg/dL decrease in nonfasting LDL levels compared with baseline. Use of simvastatin plus ezetimibe produced an additional 18-mg/dL decrease in LDL levels (an incremental 21% reduction; P<0.0001). No statistically significant effects of the addition of ezetimibe to simvastatin on triglyceride or HDL levels were observed. Ezetimibe was not associated with liver toxicity and did not impair absorption of fat-soluble vitamins. There were no serious adverse events caused by study treatment.
6. Sandhu S et al. systematic review, 200622
In 2006 an important systematic review and analysis was published22, including 27 randomised trials of statins (21 with data for eGFR and 20 for proteinuria or albuminuria) which reported kidney function or proteinuria in 39,704 patients, to assess the effect of statins on change in kidney function and urinary protein excretion. Medline, EMBASE, the Cochrane Central Register of Controlled Trials, conference proceedings, and authors personal files were searched. The results showed that the use of statins in 21 trials was associated with small reductions in rate of decline of eGFR (WMD 1.22 mL/minute per year; 95% CI 0.44 to 2.00), and that this effect was significant in a subgroup of trials including patients with cardiovascular disease (difference 0.93 mL/minute per year; 95% CI 0.10 to 1.76) but not significant in subgroups with diabetic or hypertensive kidney disease or glomerulonephritis.
In meta-analysis of 20 trials the standardised mean difference for the reduction in albuminuria or proteinuria as a result of statin therapy was significant (0.58 units of SD greater in statin recipients; 95% CI 0.17 to 0.98). The authors conclude that statin therapy seemed to reduce proteinuria modestly and resulted in a small reduction in the rate of kidney function loss, especially in populations with cardiovascular disease.
7. Douglas K et al. systematic review, 200623
In the same year as the previous one another systematic review was published23, including 15 randomised placebo-controlled trials with an average duration of 24 weeks with a total of 1,384 patients (11 trials reported albuminuria and 4 trials reported proteinuria). This -analysis had some statistical heterogeneity. This was evident only in the group with excretion greater than 300 mg/d (excretion <30 mg/d, I2=23% P=0.27; excretion 30 to 299 mg/d, I2=0% P=0.64; excretion ≥300 mg/d, I2=63% P = 0.020). Statin use was associated with reduction in albuminuria or proteinuria in 13 of 15 trials.
The reduction in excretion demonstrated in trials with greater baseline albuminuria or proteinuria was as follows. No reduction (estimated +2%, 95% CI -32% to +35%) for 3 trials with baseline excretion < 30 mg/day; 48% reduction (95% CI -71% to -25%) for 6 trials with excretion 30-300 mg/day and 47% reduction (95% CI -67% to -26%) for 6 trials with excretion > 300 mg/day. The authors concluded that statins may have a beneficial effect on pathological albuminuria.
8. Post-hoc analysis of the JUPITER trial24
The JUPITER trial29, a study into 17,802 men and women with LDL<130 mg/dl but elevated C-reactive protein (CRP) (≥2 mg/l) during a median follow-up of 1.9 years (maximum 5 years), demonstrated a 44% reduction in major vascular events and a 20% reduction in all-cause mortality for those allocated to rosuvastatin 20 mg daily as compared with placebo. A secondary analysis of this trial24 evaluated the efficacy of statin therapy in primary prevention among 3,267 individuals with moderate CKD (defined as an eGFR <60 ml/min/1.73 m2), comparing cardiovascular and mortality outcomes with patients with baseline eGFR ≥ 60 ml/min/1.73 m2 (n=14,528).
Results showed that when compared with participants with higher eGFR, those with moderate CKD had higher vascular event rates (HR: 1.54, 95% CI 1.23 to 1.92, P=0.0002) and that rosuvastatin was associated with a 45% reduction in risk of a composite outcome of myocardial infarction, stroke, hospital stay for unstable angina, arterial revascularization, or confirmed cardiovascular death (HR: 0.55, 95% CI 0.38 to 0.82, P=0.002) and a 44% reduction in all-cause mortality (HR: 0.56, 95% CI 0.37 to 0.85, P=0.005). Patients had median reductions of LDL and CRP, as well as side effects, similar to the ones with CKD, with a marginal improvement in median eGFR at 12 months (Table II).
Table II
Outcomes among those allocated to rosuvastatin or placebo (eGFR<60 ml/min/1.73m2)

The authors concluded that statin therapy with rosuvastatin reduced first cardiovascular events and all-cause mortality among men and women with LDL <130 mg/dl, elevated CRP, and moderate CKD.
9. Navaneethan SD et al. Cochrane SR 200925
A Cochrane systematic review was published in 200925 that included 26 randomised placebo-controlled trials evaluating statins in 25,017 adults with CKD not requiring dialysis. The studies had methodological limitations: unclear or inadequate concealment of allocation in 21 trials; only double masking – patients and investigators – in eight trials; triple masking (patients, investigators and outcome assessors) only in one trial and no use of intention-to-treat analysis in 21 trials.
The results showed that when comparing statins with placebo all-cause mortality was reduced: 7.6% vs. 9.4% (21 trials with 18,762 patients – p <0.0001, NNT=56), with these results primarily from a single large trial with 16,824 patients; cardiovascular mortality was reduced from 4.5% to 5.7% (20 trials with 18,746 patients – p =0.00034, NNT=84), with results primarily from the same single large trial with 16,824 patients and nonfatal cardiovascular events were reduced – 14.5% vs. 18.5% – in analysis of 5 trials with 19,363 patients (p < 0.00001, NNT 25).
In this review statins were associated with decreased total cholesterol in analysis of 18 trials with 1,677 patients (WMD=-41.5 mg/dL, 95% CI -50 to -34 mg/dL); decreased low-density lipoprotein (LDL) cholesterol in analysis of 16 trials with 1,605 patients (WMD=-42.4 mg/dL, 95% CI -50.7 to -34.1 mg/dL, P <0.00001); decreased 24-hour urinary protein excretion in analysis of 6 trials with 311 patients (WMD=-0.73 g/24 hours, 95% CI -0.95 to -0.52 g/24 hours, P<0.00001). No significant differences in creatinine clearance were detected in 11 trials with 548 patients. Also, no significant differences in incidence of rhabdomyolysis, elevated liver enzymes or withdrawal rates due to adverse events were found.
10. SHARP trial26
The largest clinical trial published to date directly modulating lipids in CKD was the SHARP (Study of Heart and Renal Protection) trial, performed in 380 hospitals in eighteen countries26 . The clinical question was the efficacy and safety of simvastatin plus ezetimibe in patients with chronic kidney disease (32.6% on dialysis). It was a randomised placebocontrolled trial with concealed allocation, triple blinding (patients, clinicians, and outcome adjudicators), with a follow-up ≥4 years (median 4.9 years for survivors). The sample included 9,270 patients with a mean age of 62 years (63% men) who had CKD, defined as a previous serum or plasma creatinine level ≥1.7 mg/dL in men or 1.5 mg/dL in women and no previous acute myocardial infarction or coronary revascularisation. Patients with low compliance with placebo during the six-week run-in period were excluded. Patients were randomly allocated to simvastatin, 20 mg/day, plus ezetimibe, 10 mg/day (n=4,650), or placebo (n=4,620). 886 of 1,054 patients who were initially randomly assigned to simvastatin alone were re-randomised after one year to simvastatin plus ezetimibe or placebo, and are included in the samples above.
The outcomes were: 1) first major atherosclerotic event (nonfatal AMI or coronary death, non-haemorrhagic stroke, or arterial revascularisation excluding dialysis access procedures); 2) major vascular event (nonfatal AMI or any cardiac death, any stroke, or arterial revascularisation excluding dialysis access procedures), and 3) end stage renal disease (ESRD).
Final follow-up was 98% (intention-to-treat analysis). The table shows that simvastatin + ezetimibe reduced risk of major atherosclerotic events and major vascular events more than placebo. In patients who were not receiving dialysis at the start of the trial (n=6,247), groups did not differ for ESRD.
Comparing ezetimibe/simvastatin combination vs. placebo, nonfatal myocardial infarction was 2.9% versus 3.4% (not significant), coronary heart disease mortality was observed in 2% vs. 1.9% (not significant), non-haemorrhagic stroke in 2.8% vs. 3.8% (p = 0.01, NNT 100) and revascularisation in 6.1% vs. 7.6% (p = 0.0036, NNT 67). The conclusions of this study were that the combination simvastatin plus ezetimibe reduced risk for major atherosclerotic events in patients with CKD.
Table III
Lipid modulation in CKD

CONCLUSIONS
The best published studies show that CKD is a powerful risk factor for cardiovascular disease, with event rates which remained 25% higher than those who had normal kidney function. Our paper confirms the benefit of statins in patients with moderate CKD.
In short, there are a series of statements that can be made concerning the effects of statins in patients with CKD not on dialysis: The combination ezetimibe/simvastatin reduces AMI, non-haemorrhagic stroke and revascularization in these patients, and less than 50 patients need to be treated over four years to avoid a CV event.
The combination ezetimibe/simvastatin reduces LDL levels more than simvastatin alone.
Treatment with atorvastatin plus ACE inhibitors or ARBs may reduce proteinuria and the rate of progression of kidney disease, proteinuria and hypercholesterolaemia.
Pravastatin was associated with slower renal function decline that placebo in patients with moderate reduced GFR and proteinuria.
Simvastatin has similar effects on total cholesterol, LDL and triglyceride in CKD patients than in patients with normal kidney function.
Statin use is associated with reduction in albuminuria or proteinuria.
Statin therapy with rosuvastatin reduced first cardiovascular events and all-cause mortality among men and women with low LDL, elevated CRP and moderate CKD.
Pravastatin may not be superior to usual care in preventing end-stage renal disease.
Addition of atorvastatin to angiotensin-converting enzyme inhibitor or angiotensin receptor blocker may slow progression of renal disease.
References
1 Foley RN, Murray AM, Li S, et al . Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005 ;16:489-495 [ Links ]
2 Go AS, Chertow GM, Fan D, et al . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004 ;351:1296-1305 [ Links ]
3 Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003;42:677-684 [ Links ]
4 Reinecke H, Trey T, Matzkies F, Fobker M, Breithardt G, Schaefer RM. Grade of chronic renal failure, and acute and long-term outcome after percutaneous coronary interventions. Kidney Int 2003;63:696-701 [ Links ]
5 Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 2007 ;167:2490-2496 [ Links ]
6 Matsushita K, van d, V, Astor BC, et al . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative-analysis. Lancet 2010 ;375:2073-2081 [ Links ]
7 Strippoli GF, Navaneethan SD, Johnson DW, et al . Effects of statins in patients with chronic kidney disease: -analysis and -regression of randomised controlled trials. BMJ 2008 ;336:645-651 [ Links ]
8 Khan SS, Kazmi WH, Abichandani R, Tighiouart H, Pereira BJ, Kausz AT. Health care utilization among patients with chronic kidney disease. Kidney Int 2002 ;62:229-236 [ Links ]
9 BaigentC, Blackwell L, Emberson J, et al . Efficacy and safety of more intensive lowering of LDL cholesterol: a-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-1681 [ Links ]
10 Wanner C, Krane V, Marz W, et al . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005 ;353:238-248 [ Links ]
11 Fellstrom BC, Jardine AG, Schmieder RE, et al . Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009 ;360:1395-1407 [ Links ]
12 Holdaas H, Fellstrom B, Jardine AG, et al . Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 2003 ;361:2024-2031 [ Links ]
13 Nogueira J, Weir M. The unique character of cardiovascular disease in chronic kidney disease and its implications for treatment with lipid-lowering drugs. Clin J Am Soc Nephrol 2007 ;2:766-785 [ Links ]
14 Yamamoto S, Kon V. Mechanisms for increased cardiovascular disease in chronic kidney dysfunction. Curr Opin Nephrol Hypertens 2009;18:181-188 [ Links ]
15 Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Int Med 2004 ;140:9-17 [ Links ]
16 Contreras G, Hu B, Astor BC, et al. Malnutrition-inflammation modifies the relationship of cholesterol with cardiovascular disease. J Am Soc Nephrol 2010;21:2131-2142 [ Links ]
17 Bianchi S, Bigazzi R, Caiazza A, Campese VM. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am J Kidney Dis 2003 ;41:565-570 [ Links ]
18 Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003 ;14:1605-1613 [ Links ]
19 Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria.Circulation 2004 ;110:2809-2816 [ Links ]
20 Baigent C, Landray M, Leaper C, et al . First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis 2005;45:473-484 [ Links ]
21 Landray M, Baigent C, Leaper C, et al . The second United Kingdom Heart and Renal Protection (UK-HARP-II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. Am J Kidney Dis 2006 ;47:385-395 [ Links ]
22 Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a-analysis. Journal of the American Society of Nephrology 2006 ;17:2006-2016 [ Links ]
23 Douglas K, OMalley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Int Med 2006;145:117-124 [ Links ]
24 Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010 ;55:1266-1273 [ Links ]
25 Navaneethan SD, Pansini F, Perkovic V, et al . HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009 ;CD007784 [ Links ]
26 Baigent C, Landray MJ, Reith C, et al . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181-2192 [ Links ]
27 Sacks FM, Pfeffer M, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009 [ Links ]
28 Rahman M, Baimbridge C, Davis BR, et al . Progression of kidney disease in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin versus usual care: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Am J Kidney Dis 2008 ;52:412-424 [ Links ]
29 Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008 ;359:2195-2207 [ Links ]
António Vaz Carneiro, MD, PhD, FACP
Centro de Estudos de Medicina Baseada na Evidência
Faculdade de Medicina da Universidade de Lisboa
Av. Prof. Egas Moniz
1649-028 Lisbon, Portugal
Email: avc@fm.ul.pt
Conflict of interest statement.None declared.
This article was funded by the University of Lisbon School of Medicine.
Received for publication: 17/01/2012
Accepted in revised form: 10/02/2012














