Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.2 Lisboa abr. 2012
Prognostic factors in adult patients with idiopathic IgA Nephropathy
Cristina Silva1, Nuno Afonso1, Patricia Cotovio1, Maria Marques1, Fernanda Carvalho2, Armando Carreira1
1 Department of Nephrology, Centro Hospitalar de Coimbra. Coimbra, Portugal.
2Department of Nephrology, Hospital Curry Cabral. Lisbon, Portugal.
ABSTRACT
Introduction. IgA nephropathy is the dominant primary glomerular disease found throughout the majority of the worlds developed countries. Accurately identifying patients who are at risk of progressive disease is challenging. We aimed to characterise clinical and histological features that predict poor prognosis in adults.
Patient and Methods. We performed a single-centre retrospective observational study of biopsy-proven IgA nephropathy. The primary outcome was renal survival and death from any cause, and the secondary outcome was proteinuria remission.
Results.Data from 49 cases were available for analysis with a median follow-up of 4 years. There were no deaths.
Univariableanalyses identified acute renal failure, low estimated glomerular filtration rate for ≥3 months (low eGFR), arterial hypertension, baseline proteinuria, glomerular sclerosis >50% and interstitial fibrosis >50% as poor prognostic markers. Low eGFR persisted significant by multivariable model that used only clinical parameters. Multivariable models with histopathologic parameters observed that tubular atrophy/interstitial fibrosis >50% was independently associated with the primary outcome. Proteinuria remission throughout follow-up had no prognostic value in our revision.
Conclusions.Two independent predictors of poor renal survival at time of biopsy were found: low eGFR and tubular atrophy/interstitial fibrosis >50%.
Key-Words:IgAnephropathy; prognostic factors.
INTRODUCTION
IgAnephropathy (IgAN) is the most common primary glomerulonephritis in countries where screening for kidney disease and renal biopsy are widely practiced. Disease prevalence accounted for 17.2, 34.5, 34.9, 35.2 and 38.8% of all primary glomerulonephritis reported from Spain, Czech Republic, Finland, Italy and Northern Ireland respectively. Conversely, IgAN is not commonly reported in Africans. The reported incidence of IgAN ranged from 2.5 to 5.8% of all primary glomerulonephritis in Senegal, Sudan and South Africa1. No clinical pattern is pathognomonic of IgAN and a large cohort of undiagnosed latent IgAN is present in the general population.
Although this disorder was initially thought to carry a relatively benign course, it is estimated that 1% to 2% of all patients with IgAN will develop end-stage renal disease(ESRD) each year from the time of diagnosis2. The incidence of ESRD is 10 percent at 5 years, and this percentage increases to 15, 20 and 30 percent at 10, 15 and 20 years of diagnosis respectively3.
Identification of clinical and histological features that predict poor prognosis is important for patient therapy selection.
PATIENTS AND METHODS
Patient identification
Patients were identified from a database of renal biopsies performed in native kidney, in South Coimbra Health Region, Portugal. All patients had a biopsy demonstrating IgAN in the 20-year period between 1990 and 2010. Henoch-Schönlein purpura was excluded, as were other systemic diseases associated with mesangial IgA deposition: systemic lupus erythematosus, liver cirrhosis, hepatitis, HIV disease.
A total of 63 patients with IgAN were identified from the renal biopsy database of 402 cases. Those with secondary causes of mesangial IgA deposits were excluded: alcoholic cirrhosis (N=3), Hepatitis B (N=3), Henoch-Schönlein purpura (N=2), systemic lupus erythematosus (N=1), HIV infection (N=1) and ulcerative colitis (N=1). Three patients had missing follow-up data and were excluded as they were assumed not to have reached the primary outcome.
Data from 49 cases were available for analysis with a median follow-up of 4 years and an interquartile range of 1 to 10.8 years.
Histopathology
IgAN in the native kidney is defined as dominant or co-dominant staining with IgA in glomeruli by immunofluorescence or immunoperoxidase. The intensity of IgA staining should be more than trace and the distribution should include presence in the mesangium, with or without capillary loop staining, excluding a pure membranous, diffuse, global granular glomerular basement membrane (GBM) staining pattern or a linear GBM staining pattern.
IgG and IgM may be present, but not in greater intensity than IgA, except that IgM may be prominent in sclerotic areas. Complement C3 may be present 4.
All histopathologic samples were reviewed and scored by a single renal pathologist of the Renal Morphology Laboratory of the Curry Cabral Hospital, Lisbon, Portugal.
Pathology variables assessed were mesangial hypercellularity, endocapillary hypercellularity, extracapillary lesions (cellular, fibrocellular and fibrous crescent), glomerulosclerosis, adhesions, tubular atrophy, interstitial fibrosis, acute tubular injury and arteriolar hyalinosis.
Continuous parameters ( e.g.interstitial fibrosis) were estimated visually. A semi-quantitative histological scoring was used in glomerulosclerosis (segmental and/or global), tubular atrophy, interstitial fibrosis and arteriolar hyalinosis: classified as absent (0%), mild (1-24%), moderate (25-50%) or severe (>50%). Glomerulosclerosis and adhesions were summed for statistical power, as were interstitial fibrosis and tubular atrophy.
The percentage of glomeruli showing mesangial hypercellularity was simplified to ≤50 or >50%. Endocapillary hypercellularity, extracapillary proliferation and acute tubular injury were categorised as either present or absent.
Clinical data
Clinical characteristics were obtained from the patients nephrology clinic chart. Serum creatinine measurement and 24-hour urine protein taken immediately before the renal biopsy were used as baseline values. Estimated glomerular filtration rate (eGFR) was calculated using the four-variable MDRD formula. An eGFR <60 mL/min/1.73 m2 for ≥3 months was considered low eGFR. Values of eGFR were distributed within stages of the Kidney Disease Outcomes Quality Initiative (KDOQI) classification of chronic kidney disease (CKD). Arterial hypertension was defined as blood pressure ≥140/90 mmHg or antihypertensive medication prescription.
Children were subjects aged <12 years at biopsy.
Treatment modalities at the time of renal biopsy and/or during follow-up were classified as angiotensin-converting enzyme inhibitor and/or angiotensin receptor blockers, fish oil, tonsillectomy and immunosuppressive agents.
Outcome data were obtained from the nephrologists follow-up consultations and/or hospital stay reports.
Primary outcomes were (1) renal survival: ≥ 50% reduction from baseline eGFR or end-stage renal disease (ESRD), defined as an eGFR <15 mL/min/1.73 m2, permanent haemodialysis, peritoneal dialysis or renal transplantation and (2) death from any cause.
Patients were stratified into group I or II if they did not reach or reached the primary outcome, respectively.
Secondary outcomes analysed were achievement of a complete or partial remission. Complete remission was defined as proteinuria <0.3 g/day, while a partial remission was defined as ≥50% reduction in protein excretion from the peak value (with proteinuria ≥0.3 g/day).
Statistical analysis
Parametric variables were expressed as mean ±standard deviation (SD) and were compared using Students t-test.
Non-parametric variables were expressed as median and interquartile range (IQR) and compared using Mann-Whitney and Spearman tests. Categorical variables were expressed in percentages and were compared using Chi-square test.
Fishers exact test was used on a 2x2 table with ≥1 cells with an expected frequency ≤5. The prognostic significance of clinical and histopathologic parameters was determined using Kaplan-Meier survival analysis, univariate and multivariate Cox proportional hazards models. All p-values were two-tailed and values <0.05 were considered statistically significant. Confidence intervals included 95% of predicted values. Analyses were carried out using SPSS software (version 17; SPSS Inc. Chicago IL, USA).
RESULTS
Population
Baseline clinical features and treatment modalities at the time of renal biopsy are given in Table I. All patients were Caucasian and no children were included in the study.
Table I
Baseline clinical characteristics and treatment modalities
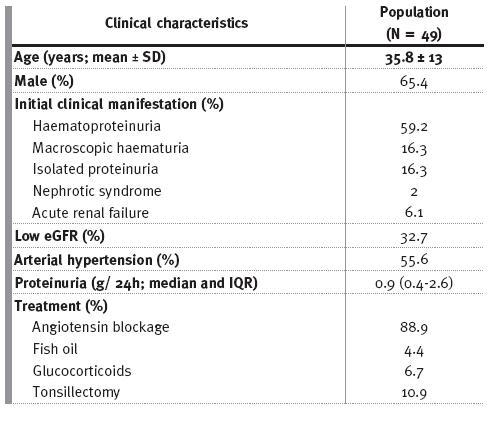
At presentation, there were 16 (32.7%) patients with low eGFR, with one patient already on ESDR. Two patients presented acute renal failure (without gross haematuria) as the initial manifestation of IgAN; both went on to ESRD years later (median time 3.8 years). A third patient developed acute renal injury during an episode of gross haematuria that required temporary dialysis; the renal biopsy revealed acute tubular necrosis and there was complete recovery of renal function thereafter.
Renin-angiotensin system blockade (RAS) consisted of angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker. Fish oil was used in combination with RAS blockade in 4.3% patients. Patients with synpharyngitic haematuria following recurrent tonsillitis went on to a consultation with an otolaryngologist, and 10.4% of these underwent tonsillectomy. Glucocorticoids were the only immunosuppressive medication used; two patients with persistent proteinuria >1 g/day (despite optimised RAS blockade and fish oil therapy) without low eGFR and a third patient, an adolescent with acute onset nephritic syndrome and severe azotaemia.
Primary Outcome
Ten patients (20.4%) developed ESRD, none presented with a ≥50% reduction from baseline eGFR or died. The actuarial renal survival from the time of renal biopsy was 76% at five-years.
Univariable model
Clinical characteristics and treatment modalities
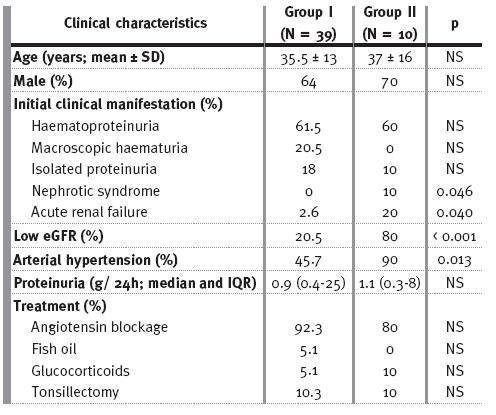
Baseline clinical characteristics of groups I and II and treatment modalities at time of biopsy and/or during follow-up are shown in Table II. About 50% of patients in group II had proteinuria =1 g/L, 30% of them in the nephrotic range.
Table II
Baseline clinical characteristics and treatment modalities
Histopathologic features
The renal biopsies of group II had significantly more glomerulosclerosis/adhesions >50%, interstitial fibrosis/tubular atrophy and endocapillary hypercellularity (Table III). A correlation between glomerulosclerosis/adhesions and interstitial fibrosis/tubular atrophy (p <0.001, r2=0.508) was observed.
Table III
Baseline histopathological features
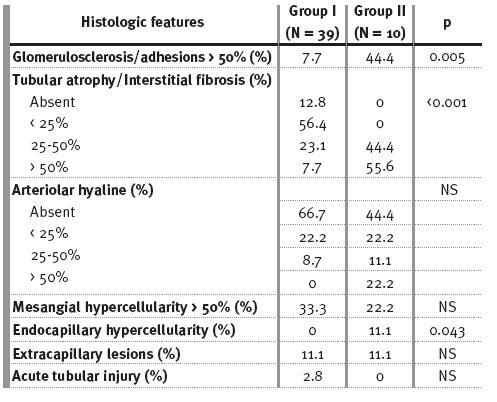
There was a significant association by univariate analysis between proteinuria at time of biopsy and glomerulosclerosis/adhesions ( p=0.044). Univariate survivorship analysis identified acute renal failure ( p=0.019), low eGFR (p<0.001), arterial hypertension ( p=0.007), baseline proteinuria (HR 1, 95% CI 1-1, p=0.009), glomerulosclerosis/adhesions >50% (p=0.001) and tubular atrophy/interstitial fibrosis >50% (p<0.001) as poor prognostic markers.
Multivariable model
Clinical characteristics
The multivariable model with clinical predictor variables significant by univariate survival analysis (acute renal failure, low eGFR, arterial hypertension and baseline proteinuria) revealed that patients with low eGFR were at higher risk of progressing to ESRD (Fig. 1).
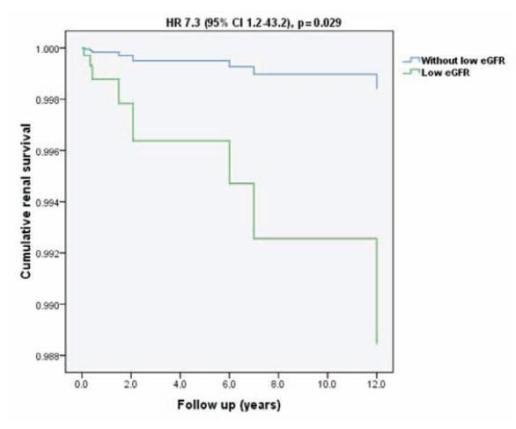
Figure 1
Renal survival plot for Cox regression in a multivariate model adjusted for clinical parameters (acute renal failure, low eGFR, arterial hypertension and baseline proteinuria)
Histopathologic features
The multivariable model with significantly histopathological parameters by univariate survival analysis (glomerulosclerosis/adhesions >50% and tubular atrophy/interstitial fibrosis >50%) observed that tubular atrophy/interstitial fibrosis >50% was independently associated with the primary outcome (Fig. 2).
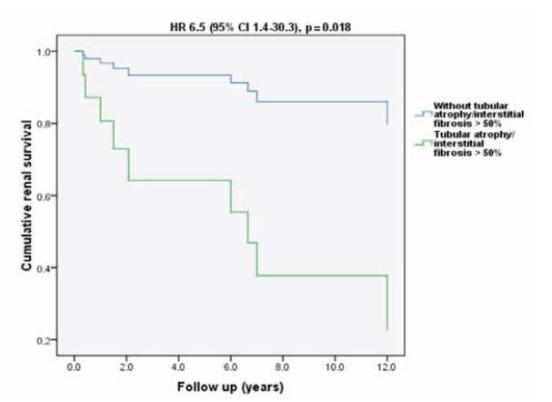
Figure 2
Renal survival plot for Cox regression in a multivariate model adjusted for histopathological parameters (glomerulosclerosis/adhesions >50% and tubular atrophy/interstitial fibrosis >50%)
Clinical and histopathologic features
The full model including both clinical and histopathologic parameters had no statistical power.
Secondary outcomes
Proteinuria throughout follow-up did not differ between groups (Fig. 3). Complete and partial remission occurred in 28.2% and 33.3% of patients in group I respectively, versus 25% and 37.5% patients in group II.
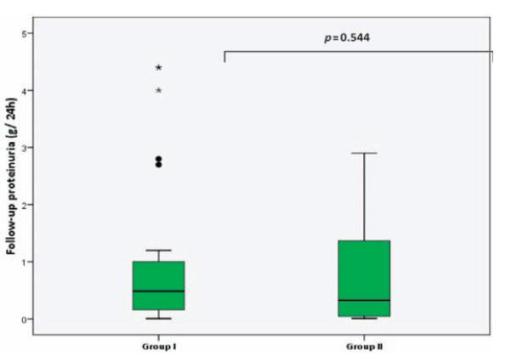
Figure 3
Boxplot of follow-up proteinuria (each box shows the median, IQR and extreme values within groups)
DISCUSSION
IgAN is the most common form of primary glomerulonephritis in developed countries5-7. Variations in disease prevalence may in large part reflect regional differences in screening for kidney disease and kidney biopsy practices7,8.
The outcome is extremely variable and difficult to predict3. Untreated adult patients with idiopathic IgAN have an actuarial 10-year renal survival rate ranging from 80% to 85%5.
Accurately predicting which individuals will go on to develop progressive disease would allow physicians to target high-risk patients for aggressive treatment or monitoring.The possibility of early intervention is of considerable interest because there is speculation that there may be a point of no return in CKD5,9 beyond which interventions are unlikely to affect prognosis significantly. Independent clinical risk factors linked to progressive IgAN include impaired renal function at presentation, arterial
hypertension and proteinuria (especially proteinuria >1g/24 h) at presentation and during follow-up2,10-17.
Acute renal failure related with macroscopic haematuria is most likely associated with heavy glomerular haematuria leading to tubular occlusion and/or damage by red cells and not to crescentic IgAN18-20. After an episode of gross haematuria, renal function typically returns to baseline and the long-term prognosis is good18. It is important to note that acute renal failure may also occur as the initial presentation of a previously undetected CKD.
Male gender, older age at presentation, persistent microscopic haematuria and absence of any history of recurrent macroscopic haematuria lose their prognostic value by multivariate analysis in the majority of studies5.
Our study supports the value of impaired renal function (acute renal failure and low eGFR), arterial hypertension and baseline proteinuria as poor prognostic markers in univariate analysis; low eGFR persisted as predictor of an unfavorable outcome by multivariable model. Data reported to nephrotic syndrome at presentation, magnitude of proteinuria at presentation and during follow-up were not statistically significant.
Findings on renal biopsy have been associated with an increased risk of progressive disease. In multivariate analyses, the extent of glomerulosclerosis and tubulointerstitial disease are most commonly associated with a poor prognosis4,5,21. These indicators are typical of most glomerular diseases4,5,21.
The Oxford classification system of IgAN4,21 suggests that four histopathologic features correlate with renal outcomes: mesangial cellularity; segmental glomerular sclerosis; endocapillary hypercellularity; percentage of interstitial fibrosis/tubular atrophy.
Our review supports the importance of glomerular sclerosis and interstitial fibrosis as significant predictors of outcome by univariate analysis but mesangial cellularity and endocapillary hypercellularity were not significant predictors.
Our study also supports the reproducibility of quantitative reporting and the significance of glomerular sclerosis >50% and tubular atrophy/interstitial fibrosis >50% in univariate analysis. Tubulointerstitial damage remained significant by multivariate analysis. The prognostic utility of crescents, necrotising lesions and extent of arteriolar hyaline was null in our study.
Our results differ from those of The Oxford classification system of IgAN21. Discrepancy may be due to the use of different outcomes (ESRD versus slope of renal function, ≥ 50% decline of eGFR or ESRD), case mix (median followup was inferior; our patients were older and no children were incorporated; all individuals were Caucasian; there were fewer men; CKD stage 1 was more prevalent and our study did not discard patients with CKD stage 4 and 5; arterial hypertension was more prevalent and median proteinuria at biopsy was lower) and/or sample size (49 versus 265 cases).
Our study fully endorses the value of clinical features and histopathologic parameters in IgAN prognosis. The results should be interpreted within the context of the studys limitations. Continuous histopathologic parameters were estimated visually and were not re-read by the studys renal pathologist to test intra-rater consistency, nor reviewed by a second renal pathologist to assess interrater reliability. As in all observational studies, the possibility of residual confounding or misclassification of outcomes cannot be excluded.
We demonstrated in a single-centre retrospective cohort of IgAN that three clinical features at time of biopsy were strong indicators of unfavorable prognosis by univariate analysis: impaired renal function (acute renal failure and low eGFR), arterial hypertension and baseline proteinuria. Low eGFR persisted significant by multivariable model.
Estimates of two simple histopathologic parameters – interstitial fibrosis/tubular atrophy >50% and glomerulosclerosis/adhesions >50% were associated with a greater risk for ESRD. Severe tubulointerstitial damage remained significant by multivariate analysis.
References
1. Ikechi Okpechi, Maureen Duffield, Charles Swanepoel. Primary glomerular diseases: variations in disease types seen in Africa and Europe. Port J Nephrol Hypert 2012;26:25-31 [ Links ]
2. DAmico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis 1992;20:315-23 [ Links ]
3.Segarra A. Progress in understanding the pathogenesis of IgA nephropathy: New perspectives for the near future. Nefrologia 2010;30:501-7 [ Links ]
4. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009;76:546-56 [ Links ]
5.DAmico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 2004;24:179-96 [ Links ]
6.Simon P, Ramee MP, Boulahrouz R, et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int 2004;66:905-8 [ Links ]
7.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int 2004;66:920-3 [ Links ]
8. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med 2002;347:738-48 [ Links ]
9. Scho¨ll U, Wastl U, Risler T, et al. The point of no return and the rate of progression in the natural history of IgA nephritis. Clin Nephrol 1999;52:285-92 [ Links ]
10. Alamartine E, Sabatier JC, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis 1991;18:12-9 [ Links ]
11. Szeto CC, Lai FM, To KF, et al. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med 2001;110:434-37 [ Links ]
12. DAmico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 2000;36:227-37 [ Links ]
13. Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int 1991;40:1050-4 [ Links ]
14. Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy. Am J Kidney Dis 2001;38:728-35 [ Links ]
15. Ikee R, Kobayashi S, Saigusa T, et al. Impact of hypertension and hypertensionrelated vascular lesions in IgA nephropathy. Hypertens Res 2006;29:15-22 [ Links ]
16.Donadio JV, Bergstralh EJ, Grande JP, et al. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 2001;17:1197-1203 [ Links ]
17.Reich HN, Troyanov S, Scholey JW, et al; Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 2007;18:3177-83 [ Links ]
18.Packham DK, Hewitson TD, Yan HD, et al. Acute renal failure in IgA nephropathy. Clin Nephrol 1994;42:349-53 [ Links ]
19.Praga M, Gutierrez-Millet V, Navas JJ, et al. Acute worsening of renal function during episodes of macroscopic hematuria in IgA nephropathy. Kidney Int 1985;28:69-74 [ Links ]
20.Fogazzi GB, Imbasciati E, Moroni G, et al. Reversible acute renal failure from gross haematuria due to glomerulonephritis: not only in IgA nephropathy and not associated with intratubular obstruction. Nephrol Dial Transplant 1995;10:624-9 [ Links ]
21.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, et al: The Oxford classification of IgA Nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009;76:534-45 [ Links ]
Dr Cristina Silva
Rua Luciano Freire 6 r/c 1600-143 Lisboa, Portugal
Email:cristinamcsilva@gmail.com
Conflict of interest statement. None declared.
Received for publication: 24/02/2012
Accepted in revised form: 21/05/2012














