Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.3 Lisboa jul. 2012
Orthotopic liver transplantation in familial amyloidotic polyneuropathy is associated with long-term progression of renal disease
Ana Carina Ferreira1, Fernando Nolasco1, Sandra Sampaio2, Alexandre Baptista2, Pedro Pessegueiro3, Estela Monteiro1, Eduardo Barroso1
1 Transplantation Unit, Hospital de Curry Cabral. Lisbon, Portugal.
2 Nephrology Department, Hospital Distrital de Faro. Faro, Portugal.
3 Nephrology Department, Hospital do Espírito Santo. Évora, Portugal.
ABSTRACT
Orthotopic liver transplantation has become the treatment of choice for familial amyloidotic polyneuropathy.
The aims of this study were to evaluate the renal complications post orthotopic liver transplantation in familial amyloidotic polyneuropathy and their impact.
We retrospectively studied 185 recipients who underwent 217 orthotopic liver transplants. Mean age 3 6.8±9.5 years, 59% males, 14.3% with renal dysfunction pre orthotopic liver transplantation. Mean follow-up 3.6±3.7 years. Thirty-two patients died. Univariate and multivariate analysis were performed, and p<0.05 was considered significant.
Acute kidney injury occurred in 57 patients and renal replacement therapy was needed in 16/57. In multivariate analysis, acute kidney injury was correlated with development of chronic kidney disease (p<0.001).
Relating to development of chronic kidney disease, 23.5% had progress to stage 3, 6% to stage 4 and 5.1% to stage 5 d. According to Spearmen correlation, risk factors for chronic kidney disease development were age (p<0.001), renal dysfunction pre orthotopic liver transplantation (p<0.001) and acute kidney injury post orthotopic liver transplantation (p<0.001).
Mortality was correlated with age (p<0.001), retransplantation need (p=0.004), renal dysfunction pre orthotopic liver transplantation (p<0.001), acute kidney injury post orthotopic liver transplantation (p=0.04), and chronic kidney disease stage 5 (p<0.001). Using binary regression, mortality was correlated with chronic kidney disease development (p=0.02).
In conclusion, familial amyloidotic polyneuropathy patients are disposed to renal complications that have a negative impact on the survival of these patients.
Key-Words: Familial amyloidotic polyneuropathy; liver transplantation; mortality; renal dysfunction.
INTRODUCTION
Familial amyloidotic polyneuropathy (FAP) is a systemic amyloidotic disease, inherited in an autosomal dominant mode1,2. This disease is due to a mutant gene in chromosome pair 18 that codifies transthyretin (TTR) protein1,2. To date, more than 80 amyloidogenic TTR variants have been described3. In Type I (Portuguese, Swedish and Japanese variant), the most frequent form, firstly described by Corino de Andrade4,5, there is an amino acid substitution of methionine for valine at position 30 (V30M) of the TTR molecule1-3,6,7.
This amino acid substitution leads to TTR protein instability that gives rise to insoluble fibres with β -sheet structures, which are deposited in the extra-cellular matrix as amyloid aggregates. However, the mutate form is not truly necessary for the amyloid deposits, since wild type is found in senile amydoidosis2.
The Portuguese type is severe, and the amyloid deposits are diffusely distributed in the peripheral nervous system of FAP patients1. The symptoms start in the lower extremities, with spontaneous pains, numbness and loss of temperature sensations that extend above ankle level. Motor deficit also occurs, with loss of balance and stepping gait3. There is a progression of the disease with a multisystemic involvement: peripheral sensorimotor polyneuropathy,autonomic dysfunction causing orthostatic hypotension, cardiac arrhythmias, gastrointestinal dysmotility, erectile dysfunction and sphincter disturbances1-3,8. Renal involvement is common. All patients have amyloid deposits in the kidney, but not all will have nephropathy1. FAP nephropathy has special epidemiological features and only one third of the patients develop chronic kidney disease (CKD) and 10% progress to CKD staged5 1.
Without treatment, the patients die after eight to ten years, following the development of muscular atrophy, severe autonomic neuropathy and malnutrition9,10.
Because more than 90% of the mutant protein is produced by the liver, orthotopic liver transplantation (OLT) has been proposed to treat this disease. The first OLT for treatment of FAP patients was performed in 1990 by Holmgren11. Follow-up of these patients was successful, and it is considered to date an effective treatment in clinical management of the disease12-14.
After OLT, kidney disease is a very important problem, and this complication significantly compromises patient outcome15-17 and increases mortality risk18-20.
Several factors, such as the presence of renal dysfunction pre OLT, the use of calcineurin inhibitors and the development of hypertension or diabetes could exacerbate kidney function15,18,19.
The aims of this study were to evaluate the incidence of renal complications post OLT in patients with FAP, to identify possible risk factors and to determine the impact on patient survival.
PATIENTS AND METHODS
Study design
This was a retrospective study of 185 FAP patients submitted to 217 OLT in our unit over a fifteen-year time period. Data were collected at day 0, 1, 7 and 21, at 6 months, 12 months and yearly after transplantation, up to the end of the study.
Clinical data included age, gender, weight, presence of diabetes mellitus, hypertension, hepatitis B and C infection, immunosuppression and need for acute renal replacement therapy (RRT). Laboratory data included serum creatinine (Scr) values and/or glomerular filtration rate (GFR), estimated by Cockcroft- Gault equation.
Renal dysfunction before transplantation (RD pre OLT) was defined by GFR ≤ 60ml/min or Scr ≥ 1.5 mg/dl. There were no records concerning proteinuria/microalbuminuria in patients clinical files.
Acute kidney injury (AKI) was defined according to the RIFLE criteria21 as a persistent decrease in renal function for at least three days using the worst value for renal function in that period.
At the end of follow-up and depending on the last value of GFR, renal function was classified according to the K/DIGO Clinical Practice Guidelines22.
Patients submitted to simultaneous liver and kidney transplants were excluded from this study.
Biochemical analysis
Biochemical analysis was performed using standard laboratory methods.
Immunosuppression protocols
Over the fifteen-year period, different immunosuppressive protocols were used. Induction protocols with ATG or basiliximab were rarely employed until 2007. Steroids were usually applied in all patients for the first months (20 mg/day), and slowly diminished up to twelve months. The association of ciclosporin andazathioprine were used in the majority of recipients up to 2003, and, since then, tacrolimus and MMF have been used. Since 2001, sirolimus has been used in a limited number of recipients. The use of ciclosporin, tacrolimus, sirolimus, azathioprine or MMF was considered as a dichotomous variable (yes or no).
Statistical analysis
Data are presented as mean±SD values for normally distributed variables or as frequencies for categorical variables. Independent variables were compared using the Mann Whitney and the chi square tests. Correlations between variables were studied with Spearman correlation test for univariate analysis, and by binary regression for multivariate analysis (confidence interval of 95%), with forward method. All tests were performed using the SPSS system 15.0 (SPSS Inc., Chicago, IL) and a p< 0.05 was considered statistically significant.
RESULTS
Table I shows the clinical characteristics of our population. Up to 2007, 217 FAP patients were transplanted in our unit, with a mean follow-up of 3.6±3.7 years, with 28.6% (n=62/217) having more than five years and 8.8% (n=19/207) more than ten years of follow-up.
Table I
Clinical characteristics of the population Renal dysfunction before transplantation
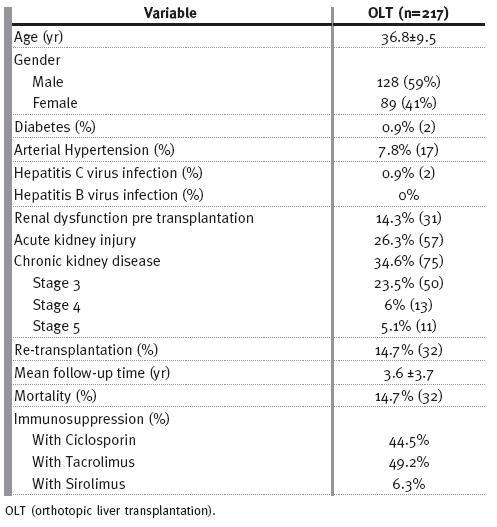
These patients were predominantly young males, with a global mean age of 36.8±9.5 years. Arterial hypertension was present in only 17 patients and a minority was diabetic (n=2) or HCV (n=2) infected patients.
There was no HBV infection among these patients. Re-transplantation was needed in 14.7% (n=32), due predominantly to thrombotic complications.
RD pre OLT was known in 14.3% (n=31) of the recipients. These patients were older (r=0.3; p<0.0001) and this finding was more frequent in women (r=0.3, p<0.0001) and in hypertensive patients (r=0.2; p=0.01). There was no correlation between the presence of RD pre OLT and diabetes or HCV infection.
Using Spearman correlation, RD pre OLT was associated with F criteria of AKI classification (r=0.2, p=0.02), CKD development (r=0.5, p<0.001): stage 3 (r=0.4; p<0.001), stage 4 (r=0.2; p=0.03), stage 5d (r=0.2; p=0.01), with RRT requirement (r=0.2; p=0.003) and with mortality (r=0.2; p=0.002). For multivariate analysis, adjusting for age, gender, and for AKI post transplant, it was significantly correlated with development of CKD (p<0.001).
Acute kidney injury (AKI)
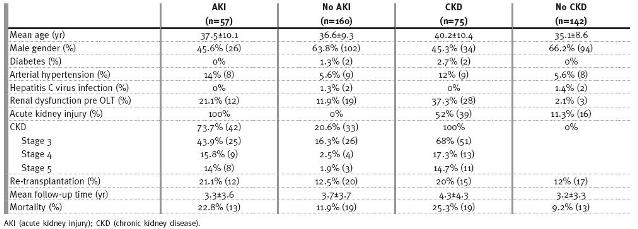
AKI was observed in 57 (26.3%) patients (Table II), predominantly in women (r=0.2; p=0.02). Acute RRT was needed in 16/57 patients (r=0.4; p<0.001). Only a minority recovered their renal function, with a GFR ≥ 60ml/min (r=-0.5; p<0.001): 25 (43.8%) developed CKD stage 3 (r=0.3; p<0.001), 9 (15.8%) developed CKD stage 4 (r=0.3; p<0.001), and 8 (14%) of these patients are on haemodialysis (r=0.2; p<0.001); 13 (22.8%) patients died (r=0.1; p=0.04) In multivariate analysis, adjusting for age and for presence of renal dysfunction before OLT, AKI correlated with development of CKD (p<0.001).
Table II
Clinical characteristics of the population according to the presence or absence of AKI or CKD
Chronic kidney disease (CKD)
According to KDOQI definitions, 34.6% (n=75/217) of the recipients developed CKD, with 51 patients (23.5%) attaining a stage 3, 13 patients (6%) a stage 4 and 11 patients (5.1%) attaining a stage 5d (Table II).
In univariate analysis, RD pre OLT (r=0.5; p<0.001) and AKI post OLT (r=0.5; p<0.001), as mentioned, were risk factors for CKD development in all three stages.
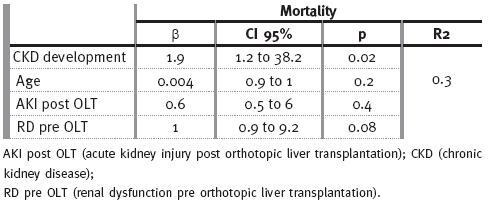
Age (r=0.3; p<0.001) was also a risk factor for CKD development, especially in stages 4 and 5d (r=0.2; p=0.003; r=0.2; p=0.008; respectively), and acute RRT requirement (r=0.4; p<0.001), specially in stage 5 (r=0.8; p<0.001). Arterial hypertension was found to be a risk factor for CKD stage 4 (r=0.3; p<0.0001), and diabetes a risk factor for stage 5 (r=0.2; p=0.001). CKD was associated with increased mortality (r=0.2; p=0.001), and this observation had particularly impact on patients with CKD stage 5d (r=0.4; p<0.001). There were no correlations between immunosuppression protocols and CKD other than a beneficial effect of tacrolimus (r=-0.2; p=0.03) on chronic renal dysfunction. In multivariate analysis, and adjusting for age, RD pre OLT, and AKI post OLT, CKD correlated with mortality (p=0.02).
By the end of follow-up, 32 (14.7%) FAP patients died, with a mean follow-up of 1.8 ± 3.2 years (r=-0.3;p<0.001). Follow-up time was shorter in older patients (r=-0.2; p=0.009) and in re-transplanted patients (r=-0.3; p=0.001).
Table III
Multivariate analysis for CKD development
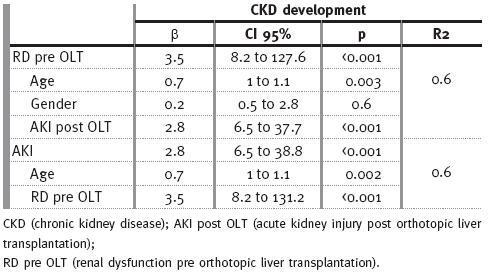
Table IV
Multivariate analysis for mortality
Mortality
According to our results, in univariate analysis mortality was associated with age (r=0.3; p<0.001) and need for re-transplantation (r=0.2; p=0.004).
Mortality was also linked to renal dysfunction, including renal dysfunction pre OLT (r=0.3, p<0.001); AKI post OLT (all classes: r=0.2; p=0.04; F class: r=0.1; p=0.03) and CKD development (all stages: r=0.2; p=0.001; stage 5: r=0.4; p<0.001).
Usingbinary regression, mortality was correlated with CKD development (p=0.02).
DISCUSSION
Liver transplantation is expected to cause a partial regression or stabilisation in the majority of symptoms in FAP patients. In fact, the neurological symptoms stabilise, but there is usually no significant improvement13. In addition, OLT must be performed early, prior to the development of malnutrition, which is associated with high morbidity and mortality10.
Nephropathy, as demonstrated by the presence of microalbuminuria or overt proteinuria, develops concomitantly with progression of the neurological disease, but occasionally may be present from the beginning and may affect one third to 75% of the patients23. Recently, it has been shown that patients with normal renal function and low levels of proteinuria have slow progression to ESRD, irrespective of the duration of their neuropathy24.
Renal dysfunction appears to be an important issue after OLT transplantation, as it is associated with higher mortality. Our study evaluates the longterm impact on renal function in 185 FAP patients submitted to 217 OLT procedures. There was a male preponderance, as is usual in FAP patients, and the mean age was 36.8 ± 9.5 years. In Portugal mean age of onset of the disease is 33.5 ± 9.5 years2 and this difference probably reflects listing time.
In our study, kidney dysfunction established by glomerular filtration rate, not by the presence of proteinuria/ microalbuminuria, was present in 14% by the time of OLT. Of note, this proportion may be certainly higher, as renal functional status in FAP patients can be underdiagnosed, due to malnutrition and reduced muscular mass, and this contributes to false lower Scr levels. However, it may be used to follow changes of renal function, as the BMI remains constant after OLT. Patients with RD pre OLT were predominantly female gender. Other groups have also shown that female gender is a risk factor for the development of CKD in FAP patients1,2. Significantly, patients presenting with RD pre OLT were at higher risk of developing AKI and CKD after OLT, and had a higher risk of mortality.
These findings impose a question: renal function pre OLT predicts an increased risk of postoperative renal failure, infection and death. This item, in many of these studied patients, is overestimated. We can conclude that in some patients a hidden risk with major consequences exists. Perhaps some patients would benefit from liver-kidney transplantation (LKTx), as haemodialysis dependence is higher in RD pre OLT patients. In this case, simultaneous LKTx has potential advantages; it avoids the progression of systemic manifestations of amyloidosis, avoids recurrence of renal amyloidosis and avoids the need for haemodialysis, providing a long-term substitution therapy for renal failure25,26. There are published articles recommending combined LKTx for CKD stage 5d in FAP TTR Val30Met25,26.
There is little published data, and appropriate criteria for simultaneous LKTx both in acute and chronic kidney diseases are being studied. The Consensus Conference on Simultaneous Liver Kidney Transplantation Review Board has proposed the following indications27:1) end stage renal disease and symptomatic portal hypertension; 2) liver failure and CKD below 30 ml/min; 3) AKI with Scr 2 mg/dl and dialysis for more than 8 weeks; 4) liver failure and CKD with more than 30% of glomerulosclerosis / fibrosis. Looking at these criteria, combined LKTx in FAP should be proposed to some patients on the topic of their renal function pre OLT. Nevertheless, it is important to note that patients with amyloidosis undergoing renal transplantation tolerate complications poorly and are at high risk of dying within the first three months, and autonomic neuropathy causing urinary retention is an additional difficulty in kidney transplantation26.
As it is a complex surgical procedure, immediate post transplantation time is crucial to its good outcome. In the long-term, hepatitis C virus infection, panel reactive antibody > 20% and female donor gender are contributing factors for unfavourable outcomes28.
AKI was observed in 26.3% of the patients after OLT. The development of AKI was a poor prognosis factor, as it was correlated with CKD. Indeed almost 74% remained with CKD.
Overall, CKD affected 34.6% patients. Hypertension, diabetes, need of RRT, RD pre OLT and AKI were risk factors for the development of different stages of CKD. Immunosuppressive protocols may impact on renal function; in reality one of the most important causes for CKD post OLT is calcineurin inhibitor toxicity, and this emphasises the need for calcineurin inhibitor minimisation protocols post transplant. In this population, mortality was correlated with CKD stage 5. Mortality was additionally correlated with age, re-transplantation and with renal dysfunction prior to OLT and with AKI, mainly with severe AKI, defined by F class.
Despite these results there are some limitations to our study. Firstly, renal dysfunction was only acceded by Scr, as there were no records regarding proteinuria, and this is an important tool to access nephropathy. Secondly this is a retrospective study, with all those drawbacks.
In conclusion, CKD development after OLT was observed in one third of our population and renal failure was associated with mortality. These results reinforce the need for early OLT for better outcome and inevitable referral to the nephrologists when there is impairment of renal function. As mortality was higher when there was renal dysfunction, we can conclude that renal complications are important prognostic tools in these patients.
References
1. Lobato L, Beirão I, Guimarães SM, et al. Familiar amyloidotic polyneuropathy type I (Portuguese): distribution and characterization of renal amyloid deposits. Am J Kidney Dis 1998;31:940-946 [ Links ]
2. Lobato L. Familiar amyloidotic polyneuropathy: how transthyretin associated amyloidosis involves the kidney. Port J Nephrol Hypert 2008;22:23-30
3. Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol 2011;10:1086-1097 [ Links ]
4. Andrade C. A peculiar form of peripheral neuropathy. Acta Psych et Neurol Scand 1951;26:251-257 [ Links ]
5. Andrade C. A peculiar form of peripheral neuropathy: familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 1952;75:408-427 [ Links ]
6. Benson MD, Uemichi T. Transthyretin amyloidosis. Amyloid 1996;3:44-56 [ Links ]
7. Hamilton JA, Steinrauf LK, Liepnieks J, et al. Alteration in molecular structure which results in disease: the Met30 variant of human plasma transthyretin. Biochim Biophys Acta 1992;1139:9-16 [ Links ]
8. Steen L, Holmgren G, Suhr O, et al. World wide survey of liver transplantation in patients with familiar amyloidotic polyneuropathy. Amyloid 1994;1:138-142 [ Links ]
9. Snanoudj R, Durrbach A, Gauthier E, et al. Changes in renal function in patients with familiar amyloid polyneuropathy treated with orthotopic liver transplantation. Nephrol Dial Transplant 2004;19:1779-1785 [ Links ]
10. Suhr O, Danielsson A, Holmgren G, Steen L. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival for familiar amyloidotic polyneuropathy. J Intern Med 1994;235:479-485 [ Links ]
11. Holmgren G, Ericzon BG, Groth CG, et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 1993;341:1113-1116 [ Links ]
12. Herlenius G, Wilczek HE, Larsson M, Ericzon BG. Ten years of international experience with liver transplantation for familiar amyloidotic polyneuropathy: results from the familiar amyloidotic polyneuropathy world transplant registry. Transplantation 2004;77:64-71 [ Links ]
13. Adams D, Samuel D, Goulon-Goeau C, et al. The course and prognostic factors of familiar amydoid polyneuropathy after liver transplantation. Brain 2000;123:1495-1504 [ Links ]
14. Yamashita T, Ando Y, Okamoto S, et al. Long-term survival after liver transplantation in patients with familiar amyloid polyneuropathy. Neurology 2012;78:637-643 [ Links ]
15. Ojo AO, Held PJ, Port FK, et al . Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931-940 [ Links ]
16. Scmitz V, Laudi S, Moeckel F. Chronic renal dysfunction following liver transplantation. Clin Transplant 2008;22:333-340 [ Links ]
17. Wilkinson A, Pham PT. Kidney dysfunction in the recipients of liver transplants. Liver Transplant 2005;11:547-551 [ Links ]
18. Ferreira AC, Nolasco F, Sampaio S, et al. Impact of renal dysfunction on liver transplantation: a retrospective study in 708 liver transplant recipients. Port J Nephrol Hypert 2010;24:45-50 [ Links ]
19. Ferreira AC, Nolasco F, Carvalho D, et al . Impact of RIFLE classification in liver transplantation. Clin Transplant 2010;24:394-400 [ Links ]
20. Pillebout E, Nochy D, Hill G, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT). Am J Transplant 2005;5:1120-1129 [ Links ]
21. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31 [ Links ]
22. Levey AS, Eckardt KU, Tsukamoto Y, et al.n Definition and classification of chronic kidney disease: a position statement from Kidney Disease Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089-2100
23. Lobato L, Beirão I, Silva M, et al. Familial ATTR amyloidosis: microalbuminuria as a predictor of symptomatic disease and clinical nephropathy. Nephrol Dial Transplant 2003;18:532-538 [ Links ]
24. Rocha A, Lobato L, Silva H. Characterization of end-stage renal disease after liver transplantation in transthyretin amyloidosis (ATTR V30M). Transplant Proc 2011;43:189-193 [ Links ]
25. Lobato L, Ventura A, Beirão I, et al. End-stage renal disease in Familial Amyloidosis ATTR Val30Met: a definitive indication to combined liver-kidney transplantation. Transplant Proc 2003;35:1116-1120 [ Links ]
26. Lobato L, Beirão I, Seca R, et al . Combined liver-kidney transplantation in familial amyloidotic polyneuropathy TTR V30M nephrological assessment. Amyloid 2011; 18 Suppl 1:185-187 [ Links ]
27. Chopra A, Cantarovich M, Brain VG. Simultaneous liver and kidney transplants: optimizing use of this double resource. Transplantation 2011;91:1305-1309 [ Links ]
28. Hibi T, Sageshima J, Molina E, et al. Predisposing factors of diminished survival in simultaneous liver/kidney transplantation. Am J Transplant 2012 Jun 8 ( Epub ahead of print) [ Links ]
Dr Ana Carina Ferreira
Hospital de Curry Cabral
Rua da Beneficência 8
1069 Lisbon, Portugal
Email: karinadacostafer@hotmail.com
Conflict of interest statement. None declared
Received for publication: 26/04/2012
Accepted in revised form: 11/08/2012














