Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.3 Lisboa jul. 2012
Gastrointestinal presentation of disseminated histoplasmosiin a transplant recipient
Cristina Outerelo1, Rita Nortadas2, Rita Gouveia1, Ana Mateus1, Pedro Cruz1, Carlos Oliveira1, Raquel B. Ilgenfritz3, Aura Ramos1
1 Nephrology Department, Hospital Garcia de Orta, Almada, Portugal
2 Intensive Care Unit, Hospital Garcia de Orta, Almada, Portugal
3 Pathology Department, Hospital Garcia de Orta, Almada, Portugal.
ABSTRACT
Histoplasmosis is a clinically relevant fungal infection in immunosuppressed patients, in whom it may present as a serious disease.
We describe the case of a renal transplant recipient who presented with malaise, dry cough, severe weight loss, abdominal pain and watery diarrhoea. The laboratory workup revealed anaemia and elevated C reactive protein. Chest X-ray showed bilateral reticulonodular infiltrates, and computed tomography scan of the chest and abdomen revealed diffuse thickening of the inter- and intralobular septs, suggestive of lymphangitic infiltration. The abdomen had multiple lymphadenopathies along the mesenteric vessels. Endoscopic evaluation disclosed disseminated ulcers throughout the entire gastrointestinal tract, and biopsies confirmed the diagnosis of histoplasmosis. Despite adequate treatment with liposomal amphotericin B, the patient had a fatal outcome.
We describe this case because of the rarity of gastrointestinal presentation in the context of disseminated histoplasmosis, and we also speculate about the transmission through the allograft. It highlights the importance of considering this once geographically limited disease in challenging cases, even in non-endemic areas.
Key-Words: Gastrointestinal tract; histoplasmosis; kidney transplantation.
INTRODUCTION
Histoplasmosis is a granulomatous disease caused by the dimorphic fungus Histoplasma capsulatum , described for the first time at the beginning of the twentieth century1. The environmental reservoir is the soil, and the disease is endemic in North and Central America, but it exists worldwide, and cases have been described in patients with no reported travel to endemic regions2.
In healthy individuals, inhalation of spores is followed by an acute self-limited pulmonary illness, whereas immunocompromised hosts may progress to acute respiratory distress syndrome or disseminated disease, which can be life-threatening. The incidence of disseminated histoplasmosis is greatest among these patients, especially those infected with human immunodeficiency virus (HIV). For solid organ transplant recipients, it is considered a rare opportunistic disease, with a low incidence even in endemic areas: 4.1 cases per 1000 solid organ transplants3.
CASEREPORT
A 46-year-old Portuguese Caucasian female with chronic kidney disease of unknown aetiology received a cadaveric kidney transplant six years before admission.
The donor was a 59-year-old female who died of intracranial haemorrhage. Our patient had a history of obesity, arterial hypertension, dyslipidaemia, deep venous thrombosis, oesophagitis and laparoscopic cholecystectomy for vesicular lithiasis. She had always lived in Portugal and had never travelled abroad during her entire life.
Immunosuppression regimen consisted of tacrolimus 0.5mg twice daily (trough level of 5-6ng/dL), mycophenolate mophetil (MMF) 250mg twice daily and prednisone 5mg daily. She had chronic allograft dysfunction, with stable creatinine values of 3mg/dL, and there were no episodes of rejection.
She presented with a three-month history of general malaise, dry cough and severe weight loss of 20 kilograms. More recently she reported abdominal pain and watery diarrhoea. She denied fever or other symptoms.
On admission, she was prostrated, pale, dehydrated and afebrile. Her weight was 68 kilograms, blood pressure 138/86 mmHg, pulse 78 beats per minute and respiratory rate 24 breaths per minute. Physical examination revealed clear lungs, normal cardiac sounds and non-tender abdomen with no palpable masses. There were no mucocutaneous lesions or palpable lymphadenopathies.
The laboratory evaluation on admission revealed haemoglobin 9 g/dL, white blood cell count 5.7×109/L (91% neutrophils, 5.3% lymphocytes), platelet count 155×109/L, blood urea 116 mg/dL, creatinine 3 mg/dL and C reactive protein 7.3 mg/ dL. Urinalysis showed leucocyturia, and urine culture identified Escherichia coli , treated with ceftriaxone.
Serologic testing for HIV, hepatitis B, hepatitis C and leishmania were all negative, as were blood and stool cultures. Serum polymerase chain reaction (PCR) for cytomegalovirus and Epstein-Barr virus were negative. Chest X-ray revealed bilateral reticulonodular infiltrates, and computed tomography (CT) scan of the chest disclosed diffuse thickening of the interand intralobular septs, suggestive of lymphangitic infiltration (Fig.1). An abdominal CT showed multiple lymphadenopathies along the mesenteric vessels.
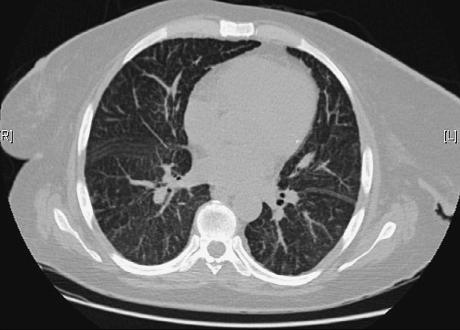
Figure 1
Chest CT scan showing diffuse thickening of interlobular septs.
Gastric and colonic endoscopic evaluations were performed, and multiple ulcers with raised borders were observed in the duodenum, colon and terminal ileum.
Biopsies taken from these ulcers revealed extensive inflammatory infiltrate, and numerous intra- and extracellular microorganisms, forming yeasts, in a diffuse histiocytosis pattern. Periodic acid-Schiff (PAS) and Grocott stains confirmed the presence of Histoplasma species (Fig.2). Serology tests were not available.
Figure 2
Colonic biopsy specimen showing intra - and extracellular organisms characteristic of Histoplasma capsulatum (Grocott stain 40×).
The patient was started on liposomal amphotericin B (3 mg/kg/day), but on the 4th day of directed therapy, she maintained anorexia, diffuse abdominal pain and abundant watery diarrhoea. By that time, progressive reduction of urinary output and worsening dyspnoea was seen. Physical and radiology examination of the lungs revealed the presence of a bilateral reticulonodular infiltrate, with a consolidation confined to the lower left lobe. She was hypotensive and hypoxaemic, so that tracheal intubation and mechanical ventilation was needed, and she was transferred to the Intensive Care Unit (ICU). Considering disseminated infection, immunosuppression was reduced: tacrolimus was targeted to a level of 3ng/dL, MMF was stopped, and steroid dose was left unchanged. She started renal replacement therapy with continuous venovenous haemodiafiltration (CVVHDF), due to oliguric acute kidney injury. Despite adequate fluid administration, vasopressors were required to restore haemodynamics. On ICU admission, it was considered that despite the diagnosis of histoplasmosis, we could not exclude hospitalacquired pneumonia, and so empiric broad spectrum antibiotic therapy with vancomycin, meropenem and gentamicin was started. Liposomal amphotericin B was continued. As she remained oliguric, dialysis dependent and there was no improvement in her clinical condition, immunosuppression was stopped. Blood, urine and bronchoalveolar secretions cultures were negative for common pathogens.
During the ICU stay, she was persistently febrile, and haemodynamically unstable. Weaning from mechanical ventilation was never successful. By the 17th day of empiric antibiotic therapy with meropenem and vancomycin (gentamicin was only administered for 5 days), as there were no isolates and the patient was not improving, antibiotics were stopped, except for amphotericin, and microbiologic tests repeated – urine, blood, and brochoalveolar secretions obtained through protected specimen brush. All the samples were once again negative.
The patient ultimately died of multiple organ failure on the 42nd day of hospital stay, the 27th in the ICU.
To our current knowledge, the receptor of the other kidney, who was transplanted in another unit, is doing well and has not had any symptoms that could be related to this disease.
DISCUSSION
Disseminated histoplasmosis among solid organ transplant recipients may occur as a primary infection, reactivation of latent infection, or donor-transmitted infection3. In immunocompromised hosts, disseminated disease may progress to septic shock, acute respiratory distress syndrome and multiorgan failure1, as occurred with our patient.
In this report, we could not confirm evidence of Histoplasma capsulatum in the lung, despite persistent pulmonary imaging abnormalities. Bronchoalveolar lavage could have been useful for confirmationof lung involvement, although finding of the fungus in organic smears is extremely difficult, even using special staining. Blood culture specific for Histoplasma growth is not routinely performed in our centre, but all the cultures sampled – blood, urine, and pulmonary secretions obtained with protected specimen brush – were persistently negative, ruling out involvement of other infectious agents.
Gastrointestinal tract involvement is common during disseminated disease (70-90%), but often remains asymptomatic4. Gastrointestinal symptoms have been described more frequently in disseminated disease affecting HIV-infected patients5.
The presence of extracellular organisms in histologic examination, as in our patient, is rare and represents a marker of severe disease4. This could explain the adverse outcome despite antifungal therapy, which has accounted for an important decrease in the mortality attributed to this infection. The majority of Histoplasma -related infections occur in the early post-transplant period, when higher doses of immunosuppression are used. In our case report, the patient had a five-year history of transplantation and her immunosuppression regimen at the time and in the past was not aggressive, which makes this case even rarer.
In 1988, the first case of disseminated histoplasmosis in Britain was reported6, in a renal transplant recipient who presented with a cecal perforation.
Since then, few case reports of transplant recipients with symptomatic gastrointestinal involvement have been described7.
Some reports seem to relate this disease in renal recipients with latent infection transmitted via the donor8,9. This is a very unlikely hypothesis, although no obvious epidemiologic context was found in the recipient. Transmission might have occurred through an infected close contact, but as a self-limited mild respiratory illness in healthy individuals, it is difficult to identify it. Also, this is an extremely rare disease in Portugal, with only a few cases described, and that explains why this diagnosis was not suspected on initial presentation.
Its rarity and nonspecific symptoms make the diagnosis and treatment difficult and often delayed, even though the prognosis appears to be good in the majority of cases described3. Our patient had a fatal outcome, which we relate to an aggressive form of disease with a long-time course.
This clinical report highlights the importance of recognising uncommon pathogens, especially as international travelling increases and immunosuppression becomes more potent. Healthcare providers must be aware of this and expect to encounter oncerare and geographically limited diseases.
References
1. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007;50:115-132 [ Links ]
2. Lo MM, Mo JQ, Dixon BP, et al. Disseminated histoplasmosis associated with hemophagocytic lymphohistiocytosis in kidney transplant recipients. Am J Transpl 2010;10:687-691 [ Links ]
3. Cuellar-Rodriguez J, Avery RK, Lard M, et al. Histoplasmosis in solid organ transplant recipients: 10 Years of Experience at a Large Transplant Center in an Endemic Area. Clin Infect Dis 2009;49:710-716 [ Links ]
4. Kahi CJ, Wheat LJ, Allen MD, et al. Gastrointestinal histoplasmosis. Am J Gastroenterol 2005;100:220-231 [ Links ]
5. Suh KN, Anekthananon T, Mariuz PR. Gastrointestinal histoplasmosis in patients with AIDS: case report and review. Clin Infect Dis 2001;32:483-491 [ Links ]
6. Brett MT, Kwan JT, Bending MR. Caecal perforation in a renal transplant patient with disseminated histoplasmosis. J Clin Pathol 1988;41:992-95 [ Links ]
7. Zainudin BM, Kassim F, Annuar NM, et al. Disseminated histoplasmosis presenting with ileal perforation in a renal transplant recipient. J Trop Med Hyg 1992;95:276-279 [ Links ]
8. Limaye AP, Connolly PA, Sagar M, et al. Transmission of Histoplasma capsulatum by organ transplantation. N Engl J Med 2000;343:1163-1166 [ Links ]
9. Wong SY, Allen, DM. Transmission of disseminated histoplasmosis via cadaveric renal transplantation: case report. Clin Infect Dis 1992;14:232-234 [ Links ]
Dr Cristina Outerelo
Nephrology Department, Hospital Garcia de Orta
Av. Torrado da Silva, Pragal
2801-951 Almada, Portugal
Email: crisout@netcabo.pt
Conflict of interest. None declared.
Received for publication: 12/03/2012
Accepted in revised form: 24/05/2012














