Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.1 Lisboa jan. 2013
Peritoneal dialysis–related peritonitis in 10 years: a single-centre experience
Peritonites em Diálise Peritoneal: experiência de 10 anos de um Serviço de Nefrologia
Rui Costa, Rui Castro, Luís Oliveira, Cláudia Bento, Sandra Cruz, Mónica Fructuoso, Catarina Prata, Teresa Morgado
Nephrology Department, Centro Hospitalar de Trás-os-Montes e Alto Douro. Vila Real, Portugal.
ABSTRACT
Aims: Evaluate the incidence, aetiology, predictors and outcomes of peritonitis in our chronic peritoneal dialysis (PD) patients. Patients and methods: A retrospective observational study was carried out in 59 patients during a ten-year period, which included the epidemiology, clinical presentation, microbiology profile, treatment and outcomes of all peritonitis episodes. Results: A total of 88 peritonitis occurred in 31 patients (mean age 43 ±15 years), with a peritonitis rate of 0.57 episodes.patient.year. Male gender (68.0% vs. 39.0%, p < 0.01) was associated with events. Coagulase-negative Staphylococcus was the most frequent agent (25.9%). Staphylococcus aureus peritonitis (17.0%) was associated with repeat peritonitis (44.4% vs. 14.3%, p = 0.02). Nasal carriage of this agent was found in half the patients and five (33.3%) episodes were preceded by exit-site infection. Culture negative peritonitis (15.9%) had lower initial peritoneal leukocyte count (< 1500 cell/mm3: 64.3% vs. 32.4%, p = 0.02). Eleven refractory peritonitis were reported and associated with longer time of treatment (34 vs. 20 days, p < 0.01) and hospital stay (16 vs. 5 days, p < 0.01). The peritoneal catheter was removed in eight cases. Cure was achieved in the majority of cases (n = 80; 90.9%), seven patients were transferred to haemodialysis, and one died. The technique survival rate at 36 months was 80%. Greater risk of peritonitis in the first year was identified in patients transferred from haemodialysis (OR 5.9; CI 95%: 1.2 -29.3) and male gender (OR 5.1; CI 95%: 1.02 – 25.1). Considering all PD patients, first year peritoneal dialysis episode was associated with higher risk for cumulative peritonitis (≥ 3) (OR 10.28; CI 95%: 1.27 -83.32). Diabetes and older age were not associated to higher risk of these events. Conclusion: The overall results of peritonitis episodes were satisfactory. Transfer from haemodialysis and male gender were associated with higher risk for first –year peritonitis. This early event was associated with a greater risk of occurrence of three or more peritonitis in those patients.
Key-Words: Outcome, peritoneal dialysis, peritonitis, predictors.
RESUMO
Objectivo: Determinar a incidência, etiologia, preditores e prognóstico dos casos de peritonite nos nossos doentes em Diálise Peritoneal (DP). Doentes e métodos: Um estudo observacional retrospectivo foi realizado em 59 doentes durante um período de 10 anos e englobou a epidemiologia, apresentação clínica, perfil microbiológico, tratamento e prognóstico dos nossos casos de peritonite. Resultados: Foram contabilizadas 88 peritonites em 31 doentes (idade média 43 ±15 anos), com ocorrência predominante no sexo masculino (68,0% vs. 39.0%, p < 0,01) e incidência calculada de 0,57 peritonites.doente.ano. O agente mais frequentemente identificado foi o Staphylococcus coagulase-negativo (25,9%). Encontrámos associação entre as peritonites por Staphylococcus aureus (17,0%) e a ocorrência de peritonites de repetição (44,4% vs. 14,3%, p = 0,02). Nestes casos, metade dos doentes eram portadores nasais e cinco casos (33%) foram precedidos de infecção do orifício de saída do cateter por este agente. As peritonites estéreis (15,9%) apresentaram contagem leucocitária peritoneal inicial inferior (< 1500 cell/mm3: 64,3% vs. 32,4%, p = 0,02) e as onze peritonites refractárias contabilizadas associaram -se a maior duração da antibioterapia (34 vs. 20 dias, p < 0,01), de internamento (16 vs. 5 dias, p < 0,01) e a remoção do cateter de DP foi necessária em oito casos. A cura foi atingida na maioria dos casos (n = 80, 90, 9%) mas sete doentes foram transferidos para hemodiálise e ocorreu uma morte por peritonite. A sobrevida da técnica aos 36 meses dos doentes com peritonite foi de 80%. A transferência da hemodiálise (OR 5,9; CI 95%: 1,2 -29,3) e o sexo masculino (OR 5,1; CI 95%: 1,02 – 25,1) foram os fatores de risco apurados para ocorrência de peritonite no primeiro ano da técnica. Considerando todos os doentes, o risco de peritonites cumulativas ( ≥ 3 episódios) foi superior nos doentes com episódio de peritonite no primeiro ano da técnica (OR 10,28; CI 95%: 1,27 -83,32). A idade e diabetes mellitus não conferiram maior risco para estes eventos. Conclusão: Os resultados obtidos foram globalmente satisfatórios. A transferência da hemodiálise e o sexo masculino foram identificados como fatores de risco para peritonite no primeiro ano da técnica. Este evento precoce está associado a maior risco para a ocorrência de três ou mais peritonites nestes doentes durante o tempo em DP.
Palavras-Chave: Diálise peritoneal, peritonite, preditores, prognóstico.
INTRODUCTION
Peritonitis is a serious complication of peritoneal dialysis (PD) and accounts for significant morbidity, technique failure and mortality. It may lead to a persistent loss of peritoneal function1,2.
Peritonitis rates may vary widely between centres, even in the same country or region3. Multiple factors may be implied in this variation: differences in patient training, connection methods, infection prevention protocols, or how each infectious event is recorded.
Therefore, to improve preventive measures and treatment, each PD unit should be aware of its own reality. Regular monitoring of microbiology profile and antibiotic sensitivity must be routinely reviewed to allow possible adjustments in empiric treatment protocols. An increase in culture-negative peritonitis incidence should be a warning sign to improve culture methods.
The aim of this study was to review and evaluate our own experience regarding epidemiology, clinical features, microbiological profile and treatment strategies of peritonitis to identify possible predictors and to allow an evidence-based approach.
PATIENTS AND METHODS
A retrospective observational study of 59 chronic renal patients of the PD Unit in our Nephrology Department was performed. Sixteen patients were transferred from haemodialysis mostly for vascular access failure (n = 12) or, less frequently, switch to the PD Unit was based on patient decision (n = 4).
All peritonitis episodes occurring between the 1st June 2001 and the 31st December 2011 were included.
Peritoneal catheters (coiled double cuff Tenckhoff) were implanted by minilaparotomy with antibiotic prophylaxis (cefazolin 1 gram i.v. before and on the day after surgery). All our patients underwent initially, at least, one month of continuous ambulatory peritoneal dialysis (CAPD) with Y connection systems and twin bags flush before fill.
The data were obtained by reviewing case records of all patients with and without peritonitis. Each event was registered in adequate software, allowing an electronic database. The peritonitis rate was reported as the number of episodes per patient-year and one episode per number of patients-months of follow-up. In determination of peritonitis rates in APD or CAPD, all cumulative time, in each modality, was considered, even in those patients who changed PD modality during our follow-up. Diagnosis of peritonitis was confirmed by at least two of the following criteria: abdominal pain and/or cloudy peritoneal effluent; effluent leukocyte count > 100 u/L and/or polymorphonuclear cells count (PMN) > 50% and positive culture of peritoneal fluid. The data recorded included age at the start of PD, gender, presence of diabetes mellitus (DM), cause of end stage renal disease (ESRD), transfer from haemodialysis (HD) or previous renal transplantation (RT) and time on technique at the event and at the end of follow-up. For each episode of peritonitis, data on clinical presentation, peritoneal leukocyte count (at diagnosis, three and five days after), infectious agents and antibiotic therapy were collected. Samples of effluent fluid for culture were taken in a blood-culture bottle (Bactek® until 2010, BacTAlert® since 2011).
An original episode and its relapses were considered a single episode. Repeat peritonitis was defined as episodes that occurred more than four weeks after completion of therapy of a prior episode with the same agent. Refractory peritonitis was defined as absence of microorganism eradication in cultures or failure of the effluent to clear after five days of appropriate antibiotics. Negative-culture peritonitis was defined as absence of culture results after a five-day incubation period. Previous exit-site infection was considered when it occurred in the four weeks prior to peritonitis. In Staphylococcus aureus (SA) peritonitis, nasal carriage of this agent was investigated and the positive cases treated with topical mupirocin.
Our units empirical antibiotic protocol is intraperitoneal vancomycin (1 -1.5g) every five days, plus ceftazidime (1g) every day at the long dwell, with adjustments after culture results. Cefazolin was used when there was vancomycin allergy. Data on hospital stay, catheter removal, and death was collected.
Death not related to peritonitis was censored. Non resolution was defined as either death due to peritonitis or technique failure (definite transfer to HD due to refractory peritonitis or ultrafiltration loss secondary to peritonitis).
The data obtained was submitted to statistical analysis using the Statistical Package for Social Sciences (SPSS) 18.0 for Windows (SPSS Inc., Chicago, Illinois). Statistical significance was taken below 5%.
The comparison between continuous variables was made using Students t-test. The categorical variables were compared by two-tailed chi-square test. To evaluate possible predictor factors of first-year peritonitis and multiple peritonitis (three or more episodes per patient), a multivariate analysis with a logistic regression model was performed (age ≥ 65 years, gender, DM, transfer from HD, educational level, CAPD, time on PD). The technique survival analysis was performed using the Kaplan-Meier and Cox regression models.
RESULTS
In our review, 59 patients performed PD with a total of 1892 months of follow-up. The mean age at the beginning of treatment was 44 ± 16 years, with a slight predominance of males (54.5%). Automatic peritoneal dialysis (APD) was the predominant technique (64.4%) and with longer follow-up (1352 vs.540 months in CAPD).
Peritonitis occurred in 31 patients (52.5%) with a total of 88 episodes (2.8/patient). The overall peritonitis rate in the studied period was one episode every 21 months (0.57 episodes.patient.year). No difference was found when comparing peritonitis rate in APD and CAPD (0.63 vs. 0.43 episodes. patient. year; p = ns) The first episode occurred a median 16 months after treatment [1 -46]. Peritonitis during the first year on PD occurred in fifteen patients (48.4%). Regarding the number of peritonitis per patient, 10 (32.3%) had a unique episode, four (12.9%) had two, eight (25.8%) had three and nine (29.0%) had four or more events.
Baseline characteristics of the patients with and without peritonitis are shown in Table I. The peritonitis group showed predominance in the male gender (68% vs. 39%, p < 0.01) and longer time on PD (47 vs. 16 months, p < 0.01). No significant difference was found in other variables (age, prevalence of DM, educational level, aetiology of ESRD, previous RT or HD or anuria). Drop-out of PD in the studied population occurred in 33 patients: 13 were submitted to RT, 14 were transferred to HD and six died, with no significant difference between the groups.
Comparison between the clinical characteristics of patients with three or more events (n = 17) and those with one or two (n = 14) is represented in Table II. Patients with more peritonitis episodes were mainly men (78% vs. 54%, p < 0.01), had a lower prevalence of DM (11.1% vs. 38.5%, p < 0.01) and a trend to longer time on PD (54 vs. 36 months, p = 0.06). Age, mean time to first-peritonitis, causes of drop -out and other variables studied were similar in both groups.
Cloudy peritoneal effluent (95%) and abdominal pain (93%) were the most common symptoms. Others clinical features were fever (68%) and nausea/vomiting/diarrhoea (20%). Criteria of sepsis were present in 5% of the patients. One patient had no symptoms, only a cloudy peritoneal effluent. Mean peritoneal leukocyte count at diagnosis was 3147 ± 2480 cell/mm3 (PMN 85 ± 80%), 372 ± 388 cell/mm3 (PMN 58 ± 30%) at the 3rd day and 150 ± 30 cell/mm3 (PMN 36 ± 31%) at the 5th day.
Gram-positive agents accounted for the majority of episodes (67.0%) and fungal infection did not occur. Coagulase-negative Staphylococcus (CoNS) was the commonest agent (26.1%; 0.15 episodes.patient.year), followed by Streptococcus species (19.3%; 0.11 episodes.patient.year), Staphylococcusaureus (17.1%, 0.10 episodes.patient.year) and other Gram -positive bacteria (4.5%, 0.03 episodes.patient. year). Gram -negative agents were identified in 15 cases (17.1%, 0.10 episodes.patient.year). Comparison between first (2001 -2006) and second five–year period of our study (2007 -2011) showed no significant decrease in Gram-positive peritonitis (85.7 vs. 80%, p = ns) or in CoNS -peritonitis (35.7 vs. 26.7 %, p = ns). Prevalence in Gram -negative peritonitis was also similar in both periods (14.3% vs. 20.0%, p = ns). In Staphylococcus aureus peritonitis (15 episodes in eight patients), positive nasal carriage for this agent was found in half the patients. Only five patients experienced exit -site infection-related peritonitis and all were caused by Staphylococcus aureus. Methicillin resistance was found in only four (4.5%) episodes (Staphylococcus aureus,
Empirical antibiotic treatment with intraperitoneal vancomycin and ceftazidime was performed in 84 episodes (95.6%). In the four cases of known vancomycin allergic reations, cefazolin and ceftazidime were used. After microbiology results, 51 (58%) cases were treated with vancomycin alone, 16 (18%) with vancomycin and ceftazidime, eight (9%) with ceftazidime alone, four (5%) with cefazolin alone and in nine episodes (10%) another antibiotic was used.
The duration of antibiotic therapy was 22 ± 8 days. In all cases with positive nasal carriage of SA, topic mopirucin treatment was performed.
We analysed nine (10.2%) repeated peritonitis episodes. Four (44.4%) developed within 3 months of antibiotic completion for the previous event.
Almost all episodes were caused by Gram-positive agent (88.9%), with higher percentage of SA episodes than the non-repeated peritonitis (44.4 vs. 14.3%, p = 0.02). Staphylococcus aureus peritonitis was usually treated with vancomycin for a period no less than 21 days. In only one case of repeated SA peritonitis with concomitant exit–site infection, oral rifampicin was added to IP vancomycin. No difference was found in the primary cure rate (88.9 vs. 91.1%, p = ns), hospital stay (44.4 vs. 39.2%, p = ns) or catheter removal (11.1 vs. 8.9%, p = ns).
Culture-negative peritonitis occurred in 14 (15.9%) episodes and no difference was found in baseline characteristics, exchanges with icodextrin, previous antibiotic use or in clinical evolution (Table III). However, this group had lower initial peritoneal leukocyte count (< 1500 cell/mm3: 64 vs. 32%, p = 0.02). The eleven (12.5%) refractory peritonitis cases were associated to longer length of treatment (34 ± 13 vs. 21 ± 6 days, p < 0.01), hospital stay (16.6 ± 15.0 vs. 5.7 ± 2.7 days, p < 0.01) and highest peritoneal leukocyte count at the 5th day (292 ± 198 vs. 137 ± 94 cell/mm3, p < 0.01). The baseline characteristics, time on PD, number of previous peritonitis episodes, causative microorganism (Gram-positive/Gram-negative/culture negative) or previous exit-site infection were similar to non-refractory peritonitis (Table III).
The primary cure rate was 87.5%. Hospital stay was necessary in 35 (39.7%) episodes with a mean length of 6.7 ± 5.6 days. Catheter removal was necessary in eight episodes: one repeated peritonitis caused by SA methicillin-sensitive and seven refractory peritonitis caused by Staphylococcus epidermidis methicillin -sensitive (n = 1), Pseudomonas aeruginosa(n = 1), Corynebacterium sp (n = 1), Bacillus licheniformis (n = 1) and three cases with no microorganism identification. A new PD catheter and resumption of PD was possible in three patients after short time on temporary haemodialysis.
The duration of PD varied from one to 110 months (median 27.1 months). Of the 31 patients that experienced peritonitis, one peritonitis-related death occurred and eight patients (25.8%) were permanently transferred to HD due to acute and long-term peritonitis complications (refractory peritonitis = five; ultrafiltration failure = three). The other causes of death were non-PD related infectious (n = three) and cardiovascular events (n = two). The patient survival rate at 36 months of follow-up was 87%.
Considering all technique failure cases, 61.5% were related to peritonitis complication. The technique survival rate in the peritonitis group at 36 mont of follow-up was 80% and no significant difference was found compared to non-peritonitis patients (80% vs. 100%, log rank p = ns) (fig. 1). Using the Cox regression model, no variable (age, gender, DM, transfer from HD, peritonitis in first year, patients with three or more peritonitis episodes, type of solute transport, CAPD/APD) was predictable of increased risk to technique failure.
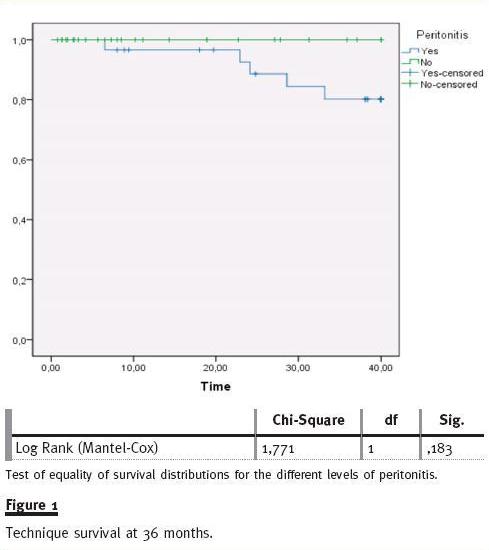
Multivariate analysis with logistic regression model was performed to analyse the effect of variables in peritonitis risk, peritonitis at first year PD and risk of three or more peritonitis episodes (Table IV). In our review, no variable was associated with an increased risk of peritonitis. Transfer from HD (OR 5.9; CI 95%:1.2 -29.3) and male gender (OR 5.1; CI 95%: 1.02 -25.1) were identified as independent predictors of occurrence of peritonitis during the first year of PD. Peritonitis in the first year of PD (OR 10.28; CI 95%: 1.27 -83.32) was associated with a higher risk of developing three or more peritonitis episodes.
DISCUSSION
In our ten-year review, the peritonitis rate was 0.57 episodes.patient.year, a result that is in agreement with the 2010 ISPD Recommendations (lower than 0.67/episodes.patient.year)4. These satisfactory results may be explained by multiple factors: adequate educational training with refreshment after each episode of peritonitis; regular domiciliary visits by the staff, the use of double cuff catheter; safer connection systems (Y set and twin bag flush before fill system); and use of prophylactic antibiotics (after aseptic technique break and during invasive procedures such as colonoscopy and cystoscopy).
In our experience, no significant difference between peritonitis in APD and CAPD was found. Criteria for selecting APD in our Unit consisted essentially on decision option or, less frequently, on clinical indication for high solute-transport patients with hypervolemia. At the time of this report, assisted PD was residual in our Unit. Results of comparative analysis in peritonitis incidence in APD and CAPD are controversial: while some studies have detected a lower incidence in patients treated with APD, others have not observed any differences between the two modes of therapy, particularly if all CAPD patients use Y set and flush before fill system5-8.
Gram-positive peritonitis accounted for 67% of al episodes in our study. In fact, most authors have described Gram-positive microorganisms as the major causative agents of peritonitis, with CoNS being the most frequent. Mujais9 reported CoNS-peritonitis in almost 30% of all episodes, comparable with our results (26.1%). However, worldwide incidence of Gram-positive peritonitis is decreasing, mainly to a decline in CoNS-peritonitis incidence after the introduction of safer connections systems and techniques, with less skin contamination10. Between the first and second five-year periods of our study, we did not observe any significant decrease in overall Gram-positive peritonitis or in CoNS-peritonitis. The absence of a significant decrease may be explained by the use of safer systems (Y-set and flush before fill) from the beginning of our PD Unit. Gram-negative peritonitis caused by bowel transmural migration or intrabdominal pathology is usually associated with the identification of anaerobic or multiple microorganisms, but none of this secondary peritonitis occurred in our review. Accordingly, we believe that our Gram-negative episodes could be linked to touch contamination.
Nasal carriage of Staphylococcus aureus is associated with increased risk of catheter exit-site infection and probably of peritonitis11. This was confirmed in our study since half of the patients with SA peritonitis were nasal carriers and five episodes (33%) were preceded by exit-site infection. During this observational period, Staphylococcus aureus nasal carriage state was performed mainly in cases of SA peritonitis (repeated or not) and in SA exit-site infection. Regular nasal swab should be routinely performed because treatment with nasal mupirocin has been demonstrated to reduce the incidence of exit-site infection12 and peritonitis incidence13. After mupirocin treatment, eradication was achieved in all our positive carriers.
Despite the fact that SA peritonitis is associated to poor prognosis14,15, only one catheter was removed in a relapse episode and no death-related peritonitis occurred in our experience.
Empirical antibiotic protocol must cover Gram-positive and Gram-negative agents4. Which drugs should be chosen depends on each centres microbiology profile. In methicillin-sensitive peritonitis, some authors found similar efficacy with empirical cefazolin compared to vancomycin (due to higher intraperitoneal concentrations), but better outcomes were achieved with vancomycin16. However, frequent empirical use of vancomycin increases the risk of antibiotic resistance, particularity of the Enterococcus and Staphylococcus species. Although an increase in methicillin-resistance prevalence is reported in many studies10,17, our results were lower than expected.
This fact raises the question whether cefazolin should be the initial choice to cover Gram–positive agents in our empirical antibiotic protocol. In our unit, no vancomycin resistance was identified. Also, thanks to vancomycins half-life, five -day intervals between administrations are possible and allowed us to administer it in subsequent follow-up out-patient appointments, with the guarantee of treatment adhesion. Since vancomycin is used in our empirical antibiotherapy protocol, increased risk of vancomycin-resistance is present. Added to this, low prevalence of methilicin-resistance prevalence was found. Those two facts made us consider a switch to cephazolin. Nevertheless, the facility on ambulatory vancomycin posology is the main reason for our doubts.
Around 10% off all peritonitis episodes were followed by repeat peritonitis, with almost half the cases in the following three months. Association of a repeated event after initial Staphylococcus aureus peritonitis was shown. This finding is similar to other published reports18. Our results indicate that repeat peritonitis deserve special attention because, although the first episode may be promptly treated, there is a substantial risk of developing repeated episodes, especially in SA peritonitis. This may indicate catheter colonisation with biofilm formation, and subsequent aggressive antibiotic treatment or catheter removal should be considered. As previously mentioned, positive nasal carriage must also be excluded.
Failure to isolate the causative agents was seen in about 16% of episodes. Isolation of the organism depends upon culture techniques, concentration of the dialysate effluent, previous antibiotic treatment and lag times between obtaining and culturing peritoneal fluid4. Culture-negative peritonitis was associated to an inferior initial leukocyte count. No difference in previous antibiotic treatment or icodextrin use was found. In our review, no specific cause for our result was identified but we believe that contribution of multiple factors may have occurred (culture performed under antibiotherapy, delay in sample processing, especially when collected at night, different blood-culture bottles used during follow-up). Additional multidisciplinary efforts between PD Unit and Microbiology laboratory on sample collection and processing are crucial to prevent further episodes with failure to isolate causative agents. Studies demonstrated that the clinical outcome in negative culture peritonitis is relatively benign19. This favourable clinical evolution was confirmed in our review.
The expected poor prognosis in refractory peritonitis was shown in our study: longer hospital stay, treatment length and peritoneal leukocyte count at 5th day were found. To preserve peritoneal membrane and improve patient outcome, catheter removal was performed in approximately two-thirds of the episodes.
In the remaining patients, despite persistent positive culture exams at the 5th day of treatment, the favourable symptomatic evolution and progressive reduction in peritoneal leukocyte count allowed us to maintain peritoneal catheter with close surveillance.
After multivariate analysis, no significant predictor factor for peritonitis was identified. Higher risk to first-year PD episode was found in male patients and in those transferred from HD. Other reports20,21 showed previous HD as a predictor variable of peritonitis, but first -year PD episode was not analysed.
Our patients had been on HD for a long time and mainly had exhausted all vascular access options.
This forced transfer may be associated to less motivation and effort in those patients, resulting in lower technical skills. The absence of residual renal function may also be an important contributor to this increased risk, since reports have shown that loss in residual renal function is an independent factor for peritonitis22. Due to the increased risk for peritonitis in HD-transferred patients, additional attention and educational reinforcement should be performed especially during the first year on PD. Male gender was also associated to a higher risk of first–year peritonitis. In previous reports that evaluated risk associated to gender, only one found an increased risk in diabetic females21. Our finding probably resulted from a selection bias, since our analysis was retrospective. Continuous ambulatory peritoneal dialysis, educational level and particularly older age or diabetes mellitus, were not predictors of first–year PD peritonitis.
In our review, the patients with peritonitis during the first year on PD were at increased risk of developing three or more peritonitis during the entire PD time. Again, multidisciplinary efforts to prevent early events and patient re -education after each peritonitis episode should be reinforced.
A satisfactory technique survival rate at 36 months was found in our review and only one peritonitis-related death occurred. We did not find any predictor of increased risk to technique failure. This lack of statistical significance may be explained by the low number of patients included and the outcomes (technique failure).
General conclusions cannot be extrapolated based on this study since it has the limitation of relying on retrospective analysis in a single centre. Also the small patient sample analysed, reducing the statistical power, is another drawback. However, it is important that each PD unit is aware of their infection results to improve outcomes.
CONCLUSION
Peritonitis remains a common complication during peritoneal dialysis. Our overall results were satisfactory, with low mortality and technique failure rates.
In our experience, transferred haemodialysis patients are at higher risk of peritonitis during the first year of PD. In addition, this first -year event may be associated with a higher risk of three or more peritonitis episodes during follow -up. Special attention should be given to prior HD patients, in order to prevent early events.
References
1. Piraino B. Insights on peritoneal dialysis-related infections. Contrib Nephrol 2009;163:161 -168 [ Links ]
2. Pérez -Fontán M, Rodriguez-Carmona A, Garcia -Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis -related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005;25(3):274-284 [ Links ]
3. Stighen AE, Barretti P, Pecoits-Filho R. Factors contributing to the differences in peritonitis rates between centers and regions. Perit Dial Int 2007; 27(Suppl 2):S281-285 [ Links ]
4. Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 Update. Perit Dial Int 2010;30(4):393-423 [ Links ]
5. De Fijter CW, Oe LP, Nauta JJ, et al. Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Ann Intern Med 1994;120(4):264-271 [ Links ]
6. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005;45(2):372-380 [ Links ]
7. Kavanagh D, Prescott GJ, Mactier RA. Peritoneal dialysis - associated peritonitis in Scotland (1999 –2002). Nephrol Dial Transplant 2004;19(10):2584 -2591 [ Links ]
8. Rodríguez -Carmona A, Pérez Fontán M, García Falcón T, Fernández Rivera C, Valdés F. A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int 1999;19(3):253-258 [ Links ]
9. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int 2006;103:S55 -62 [ Links ]
10. Zelenitsky S, Barns L, Findlay I, et al. Analysis of microbiological trends in peritoneal dialysis -related peritonitis from 1991 to 1998. Am J Kidney Dis 2000;36(5):1009-1013 [ Links ]
11. Luzar MA, Coles GA, Faller B, et al. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N Eng J Med 1990;322(8):505-509 [ Links ]
12. The Mupirocin Study Group. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. J Am Soc Nephrol 1996;7(11):2403 -2408 [ Links ]
13. Xu G, Tu w, Xu C. Mupirocin for preventing exit -site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant 2010;25(2):587 -592 [ Links ]
14. Pérez Fontan M, Rodríguez-Carmona A, Garcia -Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis -related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005;25(3):274-284 [ Links ]
15. Cunha MLRS, Montelli AC, Fioravante AM, Neves Batalha JE, Teixeira Caramori JC, Barretti P. Predictive factors of outcome following staphylococcal peritonitis in continuous ambulatory peritoneal dialysis. Clin Nephrol 2005;64(5):378 -382 [ Links ]
16. Goldberg L, Clemenger M, Azadian B, Brown EA. Initial treatment of peritoneal dialysis peritonitis without vancomycin with a once-daily cefazolin-based regimen. Am J Kidney Dis 2001;37(1):49-55 [ Links ]
17. Kim DK, Yoo TH, Ryu DR, et al. Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center´s experience over one decade. Perit Dial Int 2004;24(5):424-432 [ Links ]
18. Szeto CC, Kwan BC, Chow KM, et al. Repeat peritonitis in peritoneal dialysis: retrospective review of 181 consecutive cases. Clin J Am Soc Nephrol 2011;6(4):827-833 [ Links ]
19. Fahim M, Hawley CM, MacDonald SP, et al. Culture -negative peritonitis in peritoneal dialysis patients in Australia: predictors, treatment and outcomes in 435 cases. Am J Kidney Dis 2010;55(4):690-697 [ Links ]
20. Lobo JV, Villar KR, de Andrade Júnior MP, Bastos Kde A. Predictor factors of peritoneal dialysis -related peritonitis. J Bras Nefrol 2010;32(2):156-164 [ Links ]
21. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009;4(7):1195-2000 [ Links ]
22. Han SH, Lee SC, Ahn SV, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 2007;22(9):2653-2658 [ Links ]
Dr Rui Miguel Costa
Nephrology Department
Centro Hospitalar de Trás-os-Montes e Alto Douro
Avenida da Noruega
Lordelo 5000-508 Vila Real, Portugal
E-mail: ruimiguelccosta@gmail.com
Conflict of interest statement. None declared.
Received for publication: 12/11/2012
Accepted in revised form: 11/02/2013














