Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.2 Lisboa abr. 2013
Inhibitors of the mammalian target of rapamycin in kidney transplantation: impact of conversion on allograft and patient survival
Inibidores da mTOR em transplantação renal: impacto da conversão na sobrevida do enxerto e do doente
Ana Farinha1, Patrícia Matias2, Cristina Jorge2, Margarida Bruges2, Teresa Adragão2, Rita Birne2, André Weigert2, Domingos Machado2
1 Department of Nephrology, Centro Hospitalar de Setúbal, Portugal
2 Transplantation Unit, Department of Nephrology, Hospital de Santa Cruz, Carnaxide, Portugal
ABSTRACT
Background: Inhibitors of the mammalian target of rapamycin (mTOR) have demonstrated a similar outcome on allograft loss but its impact on patient survival has only recently been evaluated. In this outcome analysis all renal transplants performed at our unit since 1/1/2006 until 12/31/2011, were evaluated. Allograft failure and mortality were compared between patients who were converted to mTOR and those who remained on a calcineurin inhibitor (CNI). Population and Methods: We have evaluated 425 patients (mean age 46±13 years, 60.7% male, 10.1% diabetics, mean follow-up of 44 months). During this period, 123 patients were converted to mTOR: 8 included in a protocol study, 13 due to malignancy and 102 to evidence of chronic pallograft dysfunction. The remaining patients were maintained on a CNI -based regimen. We compared patient and graft survival by Kaplan-Meier analysis and p < 0.05 was considered significant. Results: During the follow-up time, 10 patients died and 28 lost their graft. Baseline characteristics were similar, except for age(converted patients were older, p = 0.012), gender (higher male percentage in mTOR group, p = 0.04) and race (Caucasians were more prevalent in mTOR arm, p = 0.012). There was no difference between the 2 groups on either graft or patient survival, even after adjustment for age, gender, and presence of diabetes mellitus or immunological risk. Moreover, in the subgroup of patients with low immunological risk, the use of mTOR was associated with a trend towards improved renal allograft survival, although not statistically significant (p = 0.06). Conclusion: Despite worse prognosis conferred by the major indications for conversion to mTOR inhibitors (malignancy or chronic allograft dysfunction) and older age, we found no difference on allograft or patient survival between the 2 groups (mTOR versus CNI -based regimen). In patients with low immunological risk, mTOR group showed a trend towards better allograft survival.
Key words: Allograft failure, calcineurin inhibitor, inhibitors of the mammalian target of rapamycin, mortality.
RESUMO
Introdução: Os mTOR mostraram-se eficazes na imunossupressão de manutenção em relação à sobrevida do enxerto, mas o seu impacto na sobrevida do doente só recentemente foi avaliado. Neste estudo analisámos todos os doentes transplantados renais na nossa unidade desde 2006/01/01 até 2011/12/31 no que respeita à falência do enxerto e à mortalidade comparando os doentes que foram convertidos para mTOR com os que permaneceram sob inibidor da calcineurina (CNI).População e métodos: Foram avaliados 425 doentes (idade média de 46 ± 13 anos, 60,7% do sexo masculino, 10,1%, diabéticos com tempo médio de seguimento de 44 meses). Durante este período, 123 doentes foram convertidos para mTOR: 8, incluídos no protocolo de um estudo, 13 por neoplasia e 102 por evidência de disfunção crônica do enxerto. Os restantes doentes foram mantidos sob CNI. A sobrevida do enxerto e do doentes foram comparados por Kaplan-Meier e p<0,05 foi considerado significativo.Resultados: Durante o tempo de seguimento, 10 doentes morreram e 28 perderam o enxerto. As características basais foram semelhantes para ambos os grupos, com excepção da idade (os doentes convertidos eram mais velhos, p = 0,012), sexo (masculino mais frequente no grupo mTOR grupo, p = 0,04) e raça (os caucasianos foram mais prevalentes no grupo mTOR, p = 0,012).Não houve diferença entre os dois grupos na sobrevida do enxerto ou do paciente, mesmo após ajuste para idade, sexo, presença de diabetes mellitus ou risco imunológico. Além disso, no subgrupo de doentes com baixo risco imunológico, o uso de mTOR foi associado a uma tendência para a melhoria da sobrevivência dos enxertos renais, embora não estatisticamente significativa (p = 0,06).Conclusão: Apesar do pior prognóstico conferido pelas principais indicações para a conversão de inibidores de mTOR (neoplasia ou disfunção crônica do enxerto) e da idade avançada, não foi encontrada diferença na sobrevida do enxerto ou do doente entre os dois grupos. Em doentes com baixo risco imunológico, o grupo mTOR mostrou uma tendência para melhor sobrevida do enxerto.
Palavras chave: Falência do enxerto, inibidor da calcineurina, inibidor da mTOR, mortalidade.
INTRODUCTION
Inhibitors of the mammalian target of rapamycin (mTOR) have emerged from the need to develop less nephrotoxic regimens for immunosuppression in kidney transplantation with the objective to promote long-term graft function.
Mammalian target of rapamycin has been identified as the principal controller of cell growth and proliferation. Sirolimus or everolimus bind the immunophilin FKBP12 and form a complex that inhibits mTOR -mediated signal transduction pathways by blocking post -receptor immune responses to costimulatory signal 2 during G0 to G1 transition and to cytokine signalling during G1 progression. They also inhibit IL-2–and IL-4–dependent proliferation of T and B cells, leading to suppression of new ribosomal protein synthesis and arrest of the G1 -S phase of the cell cycle. These anti -proliferative effects lead to their beneficial use in malignancies, but have also been associated to adverse effects like impaired wound healing, anaemia and proteinuria1 (by impaired podocyte integrity).
The early studies that have been conducted to test efficacy of mTOR inhibitors in kidney transplantation have demonstrated a lower serum creatinine and a higher estimated glomerular filtration rate2 compared with a calcineurin inhibitor (CNI)-based regimen, but have also found higher rates of biopsy -proven rejection, although this was not reflected by a worse allograft survival3 in short follow-up periods lesser than 2 years.
Cortazar et al. presented the first trial where the risk of mortality and allograft loss is studied. They found worse outcomes in patients who were converted to mTOR inhibitors from CNI-based regimens4.
The aim of this study was to evaluate in our kidney transplant population, the mid - to longterm impact of mTOR inhibitors on allograft and patient survival.
POPULATION AND METHODS
Study population
We have performed a retrospective study where we have analyzed all patients who underwent kidney transplantation, since January 1st 2006 until December 31st 2011, in our centre. We have collected information from the clinical records. Patients with incomplete information were excluded. The final sample included in 425 patients.
The following clinical data were collected: age, gender, race, aetiology of kidney failure, renal replacement therapy, modality before transplantation, donor type (living, deceased), presence of diabetes mellitus, occurrence of malignancy and recipients immunological risk. High immunological risk was defined as a panel reactive antibody (PRA) > 60%, more than one kidney transplant or no HLA compatibility.
Induction therapy was performed on the basis of the Unit protocol, depending on the immunological risk. Low risk patients were given the interleukin-2 receptor inhibitor basiliximab and the remaining received thymoglobulin.
Standard maintenance immunosuppressive regimens at enrolment included low-dose prednisone plus cyclosporine A or tacrolimus, and mycophenolate-mofetil (MMF) or azathioprine.
Our practice was to convert kidney transplant recipients to mTOR inhibitors without performing an allograft biopsy if there was clinical evidence of CNI toxicity or a history of malignancy.
Laboratoriy data collected included serum creatinine and 24-hour proteinuria measured at baseline and then in a biannual basis.
Conversion to mTOR inhibitors and Outcomes.
We divided patients in two groups: patients who were in converted to mTOR inhibitors (either sirolimus or everolimus) and patients who remained in a CNI -based regimen (either cyclosporine or tacrolimus). Immunosuppressant levels were tested in every outpatient visit and doses were prescribed to reach low C0 levels (sirolimus: 5-8ng/ml, everolimus: 5 -8ng/ml, tacrolimus: 5 -8ng/ml, cyclosporine: 80 -150 ng/ml).
We defined as primary outcomes all-cause mortality and allograft failure, defined as the need to return to dialysis. Secondary outcomes were the evolution of serum creatinine and proteinuria in each group.
Statistical analysis.
We compared baseline characteristics among users versus nonusers of mTOR inhibitors using two-sample t-tests, Wilcoxon rank -sum test, or χ2 test, as appropriate.
Survival analysis was performed with Kaplan-Meier analysis and multivariable adjusted analysis was performed using a Cox regression model. A p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics.
There were 425 patients enrolled in this study with a mean age of 46±13 years, 60.7% were male and 10.1% were diabetics. Twenty -four patients were excluded because of incomplete information. Seventy-eight (18.4%) patients were considered to have a high immunological risk. These patients received thymoglobulin as induction therapy. Table I shows the baseline characteristics of the studied population.
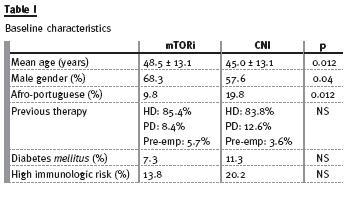
During a mean follow-up time of 44±21 months, 123 patients were converted to an mTOR inhibitor: eight included in a clinical trial, 13 with a history of malignancy and 102 because of chronic allograft dysfunction, mainly defined in the clinical setting as a progressive and slowly growing serum creatinine.
Patients were converted in a median period of 10 (1-67) months after transplantation.
Most patients were on a sirolimus based regimen (86 pts, 70%) and the remaining were on everolimus (34 pts, 30%).
Outcomes.
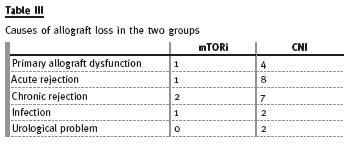
During the follow-up period, 10 patients died (table II) and 28 lost their allografts (table III). There was no statistical difference in the primary outcomes, defined as graft loss or patients mortality, in the two groups as stated in figure 1.

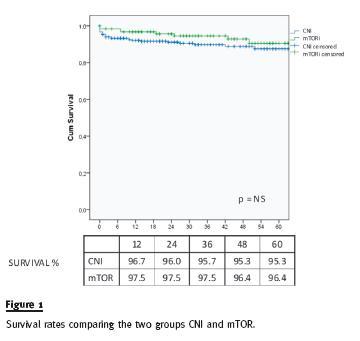
By Cox proportional hazards model, after adjustment for baseline characteristics (age, gender, race and diabetes), there was also no difference on patient and allograft survival.
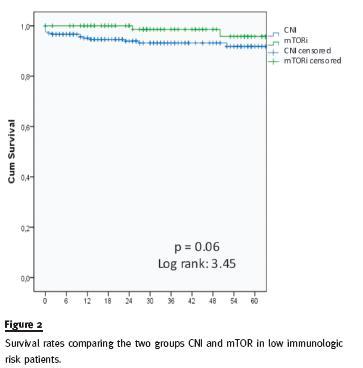
In a subgroup analysis, a trend towards a better outcome on allograft survival was seen within the 106 low immunological risk patients that were converted to mTOR inhibitor, although not statistically significant (p = 0.06), as shown in figure 2.
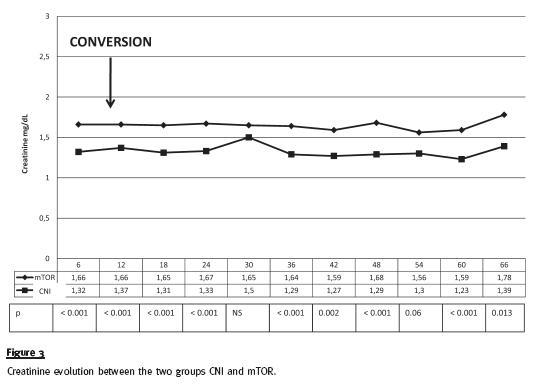
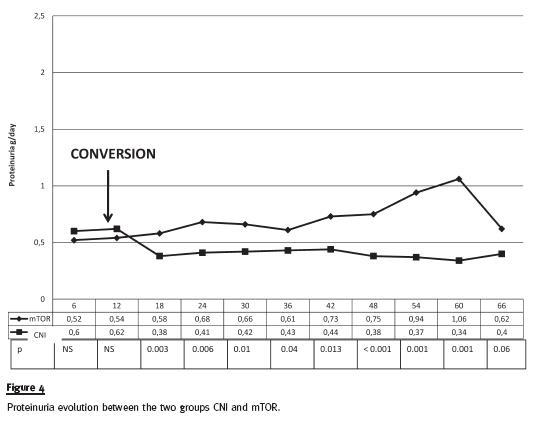
In secondary outcomes, we have observed that renal function, expressed by serum creatinine, was significantly worse in mTOR inhibitor group at the time of the conversion and, not surprisingly, serum creatinine remained stable but higher in this group of patients (figure 3). Proteinuria, at baseline was slightly, but not significantly, lower in the group of patients converted to mTOR, and as expected, there was a significant increase after conversion. In opposition, proteinuria remained stable in the CNI group (figure 4). Because of increasing nephrotic range proteinuria, oedema or severe dyslipidaemia, 18 patients have been reconverted to CNI.
DISCUSSION
In our historical cohort study with up to 6 years of longitudinal follow -up, we have found no difference on patient or allograft survival, despite the mTOR inhibitor group patients having presented worse prognostic factors, such as older age or indications for mTOR conversion (malignancy or chronic allograft dysfunction).
This is consistent with the results of other large cohort studies, which found no difference in patients who were converted from a CNI to a mTOR inhibitor. In 2006, Webster et al.2 found no difference in acute rejection, chronic allograft nephropathy, and patient or allograft survival in a meta-analysis that included 750 patients from 8 randomized controlled trials (RCT).
In 2009, Schena et al. in the Convert Trial performed a RCT with 830 patients who were converted from CNI to sirolimus in a broad post transplantation period (from 6 to 120 months). The primary end -points (graft loss or death) at 12 months were identical5.
In 2011, in the ORION study, Flechner et al. reported similar graft and patient survival in an RCT enrolling 469 patients for a 2-year follow-up period6. In the same year, Weir et al. reported a trend to a better graft and patient survival in an RCT with 299 patients, the Spare-the Nephron trial7.
Recently, in 2012, Budde et al. in the Zeus study randomized 300 patients to continue on cyclosporine or to be converted to everolimus at 4.5 months post - transplantation. At 12 months of follow -up, there was no difference in patient or graft survival8.
There are some limitations that may explain different results in different studies. The first difference stands in the time of conversion. Because of impaired healing, mTOR inhibitors have been avoided in the immediate post -transplantation period2,9. Studies where mTOR inhibitors have been introduced soon after transplant have reported worse outcomes compared to CNI10. In our study, although there has been a great dispersion in the time of mTOR conversion, it occurred in a mean period of 10 months. Second, therapeutic drug levels are important to draw conclusions.
We have used low dose regimens, perhaps limiting dose -related side effects like dyslipidaemia and anaemia associated to worse cardiovascular outcomes that would influence patient survival.
Finally, induction and concomitant therapy may also influence results. All our patients underwent induction therapy and most were maintained on MMF as anti-metabolite.
In our study, we cannot infer conclusions about mTOR inhibitors benefits in Glomerular Filtration Rate (GFR) because the main indication to switch was chronic allograft dysfunction. However, we may state that serum creatinine remained stable in the follow-up period. Proteinuria is also an independent factor for worse outcome. After an initial significant increase in proteinuria at the time of conversion, it remained stable thereafter and outcomes were not different.
In the subgroup analysis, the only difference we found was in the low immunological group, who presented a trend to a better allograft survival in comparison with the patients who remained in CNI11.
Our study presents several limitations. It is a retrospective study, with a limited number of patients. From another point of view, this study included all transplant recipients at our unit, a real life situation that may be different from many clinical trials. It is a single centre study, so results cannot be extrapolated to other populations.
Another important limitation is that the main conversions were made in the setting of chronic allograft failure but biopsies have not been obtained systematically.
The number of events is also small, a fact that may influence results, but the follow-up time, up to 72 months, is rather significant, since the literature has limited data in long follow-up periods.
CONCLUSION
We believe our results may be helpful in the decision analysis of mTOR inhibitor conversion. Until more studies are conducted, we cannot recommend or discourage conversion to mTOR inhibitors but its use seems a reasonable alternative with appropriate indications.
References
1. Merkel S, Mogilevskaja N, Mengel M, Haller H, Schwarz A Side effects of sirolimus. Transplant Proc. 2006;38(3):714 -715 [ Links ]
2. Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: A systematic review and meta -analysis of randomized trials. Transplantation 2006; 81(9):1234 -1248 [ Links ]
3. Ekberg H, Tedesco -Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357(25): 2562-2575 [ Links ]
4. Cortazar F. Molnar MZ, Isakova T, et al. Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. Am J Transpl 2012;12(2):379 -387 [ Links ]
5. Schena FP, Pascoe MD, Alberu J et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24 -mon efficacy and safety results from the CONVERT trial. Transplantation 2009;87(2):233 -242 [ Links ]
6. Flechner SM, Glyda M, Cockfield S, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011;11(8):1633-1644 [ Links ]
7. Weir MR, Mulgaonkar S, Chan L, et al. Mycophenolate mofetil -based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int. 2011;79(8):897-907 [ Links ]
8. Budde K, Lehner F, Sommerer C, et al. Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3-year results from the randomized ZEUS Study. Am J Transplant 2012;12(6):1528-1540 [ Links ]
9. Hardinger KL, Koch MJ, Brennan DC. Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy 2004;24(9):1159-1176 [ Links ]
10. Isakova T, Xie H, Messinger H, et al. Inhibitors of mTOR and Risks of Allograft Failure and Mortality in Kidney Transplantation. Am J Transplant 2013;13(1):100-110 [ Links ]
11. Morath C, Arns W, Schwenger V, et al. Sirolimus in renal transplantation. Nephrol Dial Transplant 2007; 22 (Suppl 8):viii61-viii65 [ Links ]
Ana Farinha
Department of Nephrology, Setúbal Hospital Centre
R. Camilo Castelo Branco – Apartado 140
2910 -446 Setúbal, Portugal
E-mail: alpfarinha@yahoo.com.br
Conflicts of interest statement. None declared
Received for publication: 31/01/2013
Accepted in revised form: 07/05/2013














