Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.4 Lisboa dez. 2013
ORIGINAL ARTICLE
Renal Involvement in Multiple Myeloma: an experience of a single centre
Envolvimento Renal no Mieloma Múltiplo: a experiência de um centro
Clara Santos1, Daniela Lopes1, Patricia Barreto1, Catia Cunha1, Ana Marta Gomes1, Ana Ventura1, Henrique Coelho2, Joaquim Seabra1
1Nephrology Department, Centro Hospitalar Vila Nova de Gaia. Vila Nova de Gaia, Portugal
2Haematology, Centro Hospitalar Vila Nova de Gaia. Vila Nova de Gaia, Portugal
ABSTRACT
Introduction: Multiple myeloma is a plasma cell dyscrasia that accounts for almost 10% of all haematologic malignancies. It often presents with acute kidney injury that has long been associated with a poor prognosis. It is important to recognize markers of poor prognosis to promote an early and aggressive management of the disease, to improve disease outcomes. Subjects and Methods: We have, therefore, investigated the clinical presentation and outcome of all 44 myelomas diagnosed in our hospital, comparing those with and without renal involvement and exploring factors associated with mortality over a 2-year period of time using the Cox regression method. Results: We found that the group of patients with renal disease (n = 18) were at higher stages of disease (stage III, 78% vs. 23%, p= 0.001), had higher percentage of plasma cells (≥ 15%, 72% vs. 38%, p = 0.027), higher values of B2-microglobulin (≥ 4.5mg/L, 83% vs. 35%, p = 0.001), lower values of haemoglobin (Hb < 9.5g/dL, 50% vs. 15%, p = 0.013) and lower values of albumin (< 3.5g/dL, 39% vs. 12%, p = 0.033). The most common type of renal involvement was cast nephropathy (44%). This group of patients had significantly lower survival at 12 and 24 months (75% versus 92% and 41% versus 91%). In the multivariate analysis, two factors were found to be significantly and independently associated with mortality: serum albumin < 3.5g/dL [hazard ratio 6.68, CI: 1.27-33.05; p = 0.025] and light chain MM (HR 7.34; CI: 1.63-49.4; p = 0.009). Conclusions: Renal involvement is a common complication of multiple myeloma and these patients have poor survival. Therefore, it is of crucial importance to have a high suspicion index to do an early diagnosis and to promote early and aggressive management of renal insufficiency and myeloma. However, this worse outcome seems to be related to the presence of other markers of poor prognosis, like more advanced stages of disease, higher tumour loads and lower values of haemoglobin and albumin, since renal disease was not a risk of death in the multivariate analysis.
Keywords: acute kidney injury; multiple myeloma; prognosis; survival analysis.
RESUMO
Introdução: O mieloma múltiplo é uma discrasia de células plasmocitárias, que corresponde a cerca de 10% de todas as neoplasias hematológicas. O mieloma múltiplo apresenta-se, frequentemente, com lesão renal aguda que tem sido associada a um pior prognóstico. É importante reconhecer marcadores de mau prognóstico, de forma a que um tratamento precoce e agressivo da doença permita uma melhoria da evolução e dos resultados. Material e métodos: Procedemos à investigação de todos os mielomas múltiplos diagnosticados no nosso hospital durante 2 anos (44 casos), relevando a apresentação clínica, comparando aqueles com e sem envolvimento renal e explorando fatores associados a morte durante um período de tempo, utilizando o método de regressão de Cox. Resultados: Observámos que o grupo de doentes com envolvimento renal (n = 18) estavam em estádios mais avançados da doença (estádio III, 78% vs. 23%, p = 0,001), apresentavam maior percentagem de plasmócitos na medula óssea (≥ 15%, 72% vs. 38%, p = 0,027), valores de B2-microglobulina mais elevados (≥ 4,5mg/L, 83% vs. 35%, p = 0.001), valores mais baixos de hemoglobina (Hb < 9,5g/dL, 50% vs. 15%, p = 0,013) e valores mais baixos de albumina (< 3,5g/dL, 39% vs. 12%, p = 0,033). A forma mais comum de envolvimento renal foi a nefropatia de cilindros (44%). Este grupo de doentes teve uma sobrevida significativamente inferior aos 12 e 24 meses (75% versus 92% e 41% versus 91%). Na análise multivariada, encontrámos 2 fatores preditores independentes de morte: um valor de albumina sérica < 3,5g/dL (rácio de probabilidade 6,68, IC: 1,27-33,05, p = 0,025) e o MM de cadeias leves (rácio de probabilidade 7,34, IC: 1,63-49,4, p = 0,009). Conclusões: O envolvimento renal é uma complicação comum do mieloma múltiplo e estes doentes têm uma menor sobrevida. É de crucial importância ter um alto índice de suspeição da doença para se estabelecer um diagnóstico precoce e promover um tratamento rápido e agressivo da lesão renal e do mieloma. No entanto, o pior prognóstico dos doentes com doença renal parece estar relacionado com a presença simultânea de outros marcadores de mau prognóstico, como estádios mais avançados da doença, maiores cargas tumorais e valores mais baixos da hemoglobina e albumina, uma vez que a doença renal não foi um risco preditivo de morte na análise multivariada.
Palavras-chave: análise de sobrevida; lesão renal aguda; mieloma múltiplo; prognóstico.
INTRODUCTION
Multiple myeloma (MM) is a plasma cell dyscrasia that accounts for almost 10% of all haematologic malignancies1,2. The annual incidence of MM is 5.6 per 100,000 people1, but the incidence increases with age, ranging from 1 per 100,000 for people aged 40 to 49 years to 49 per 100,000 population for those who are older than 80 years3,4. The diagnosis of MM includes the identification of clonal plasma cells in bone marrow or histologic confirmation of a plasmocytoma; a monoclonal protein in the serum or urine (unless the patient has a non-secretory myeloma, which occurs in 3% of patients) and end-organ damage evidenced by renal insufficiency, hypercalcaemia, anaemia or lytic bone lesions5,6. The diagnosis of myeloma often results from the workup of unexplained renal disease, and that is why in many cases the diagnosis is made by a nephrologist. Renal impairment is one of the major complications of MM, occurring in approximately 15% to 40% of patients7,8 and slightly less than 10% present with severe renal failure at the time of diagnosis8. Because renal impairment has been associated with shorter survival9-11, it is an important consideration in the treatment of MM. Besides renal involvement, other factors have being implied to confer a worse prognosis for MM. such as age, performance status and comorbidities10. The variables described are low serum albumin (< 3g/dL), low level of haemoglobin (< 10 g/dL), low platelet count (< 150,000/microL), higher level of beta-2-microglobulin (> 4 mg/L), high serum calcium (≥ 11 mg/dL) and bone marrow plasma cell percentage ≥ 50 percent12.Our intention in this analysis was to describe the clinical features of patients with MM at our institution, investigate if those presenting with renal involvement had a worse prognosis and explore factors that influence survival.
SUBJECTS AND METHODS
General design
Following a retrospective, observational design, we investigated the impact of selected demographic, clinical and MM-related factors in patients with MM on the outcome.
We performed an audit and reviewed the medical records of all patients with a diagnosis of MM at the Centro Hospitalar Vila Nova de Gaia. from 1 January 2010 to 31 December 2011. We analysed the presentation and outcome of 44 patients by reference to hospital records. Patients were followed until death or, if they remained alive, until the 31 December 2012.
Study population
Patients were identified in an MM patients database of the Haematology department records and crosschecked with data from the Nephrology department.
The diagnosis of MM was made if the patient fulfilled the standard criteria (see above). For the purposes of this analysis, the disease was staged according to the International Staging System (Table I)13.

The main characteristics of the study population are presented in Table II. Patients were divided based on the presence of acute kidney injury at presentation, including in group 1 the patients without renal disease and in group 2 the patients with renal disease. Acute kidney injury was defined as a plasma creatinine concentration above 1.3 mg/dL.
Study variables and laboratory methods
The primary outcome of interest was death. Information on the cause of death was sought from the patients hospital medical records. The following variables were recorded from the patients medical notes: age, gender, laboratory values at presentation (serum creatinine, albumin, B2-microglobulin, calcium, haemoglobin), myeloma type, presence of osteolytic lesions, stage and treatment given.
Strategy of analysis and statistics
The main objective of the study was to establish if renal disease was associated with a poor outcome in patients with MM. For this purpose, we analysed the main demographic, clinical, biochemical and MMrelated variables. We first compared patients with or without renal disease at presentation. We then compared survival of both groups. As a third step, we performed multivariate, adjusted estimations of the risk of mortality during follow-up.
The data obtained was submitted to statistical analysis using the Statistical Package for Social Sciences (SPSS) 20.0 for Windows (SPSS Inc., Chicago, Illinois). Statistical significance was taken below 5%. Numeric variables are presented as mean ± standard deviation, unless abnormally distributed (median with range). Continuous variables were converted to categorical variables using internationally accepted cut-off values. We used the Students t-test and Mann Whitneys tests to compare numeric variables, and the χ2 distribution and Fishers exact test to compare categorized variables. All these variables were then analyzed for their association with survival and hazard ratios for death rate were calculated using Cox regression. Significance tests were two-sided.
RESULTS
Presentation and initial management
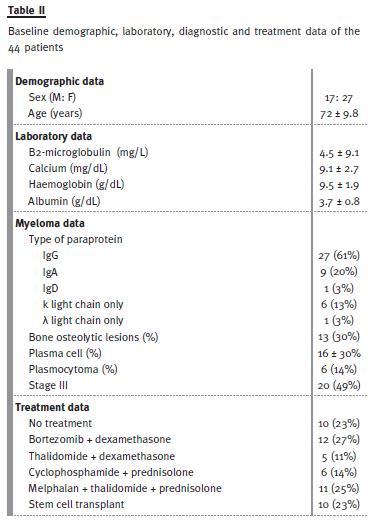
The baseline demographic, laboratory, diagnostic and treatment data of the 44 patients are shown in Table II. The mean age of patients was 72 years, with 27 females and 17 males.
More than half of the patients had IgG type myeloma, being the most frequent type, which is according to the literature12. Light chain myeloma was diagnosed in 16% of the patients (n = 7) and 49% of the patients were classified as being in stage III of the disease.
Eighteen patients (41%) had renal disease at presentation and, in 72% of these, the diagnosis was made by the Nephrology department, showing the importance of high index of suspicion.
A univariate analysis (Table III) comparing both groups showed that patients with renal involvement were at higher stages (stage III, 78% vs. 23%, p= 0.001), had higher percentage of plasma cells (≥ 15%, 72% vs. 38%, p= 0.027), higher values of B2-microglobulin (≥ 4.5mg/L, 83% vs. 35%, p = 0.001), lower values of haemoglobin (Hb < 9.5g/dL, 50% vs. 15%, p= 0.013) and lower values of albumin (<3.5g/dL, 39% vs. 12%, p= 0.033).
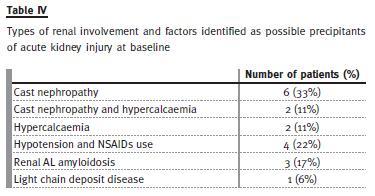
Types of renal involvement are summarized in Table IV. The most common type was cast nephropathy (8/18), in whom in two patients we also found hypercalcaemia as a contributor factor. In six patients, hypercalcaemia, hypotension and use of non-steroidal anti-inflammatory drugs were identified as the precipitants of the acute kidney injury. The diagnosis of cast nephropathy was assumed in cases of acute or rapidly progressive renal insufficiency that persisted after correction or in the absence of other potential precipitants, and proteinuria composed with light chains and not albumin. Three patients had renal AL amyloidosis and a light chain deposit disease was diagnosed in one. At presentation no patient had chronic kidney disease (renal insufficiency at least 6 months earlier). Renal biopsy was performed only in a minority of patients (4/18), either because it was considered unlikely to change the patient management in the majority of cases, or because the patient was too unwell to undergo the procedure. The histological results were: AL amyloidosis (3) and light chain deposit disease (1).
In group 2, a total of 11 patients (11/18, 61%) were dialysed during their admission or in the follow-up, at the discretion of the managing nephrologist, and the first modality was haemodialysis in all patients.
Six of the 11 patients who needed dialysis had cast nephropathy, three had acute tubular necrosis and two had AL amyloidosis. Of these, only three (17%) patients recovered sufficient renal function to discontinue dialysis, whereas the remaining patients were kept on maintenance dialysis.
The decision of how to treat the MM was made by the haematologists who reviewed all cases during the index admission. Many different chemotherapy regimens were used during the 2 years (bortezomib plus dexamethasone; melphalan plus thalidomide plus prednisolone; thalidomide plus dexamethasone; cyclophosphamide plus prednisolone). Ten patients (9%) were submitted to stem cell transplant. Ten patients (9%) were not given any chemotherapy, usually because of their advanced age and severe comorbidities. Of these, six patients were older than 80 years, three had severe cardiac disease and three others had renal involvement.
Mortality
The mean survival for the entire group was 14.4 months. Fifteen patients (34%) have died. Causes of death are summarized in Table V with more than one third of all deaths being attributed to infectious complications.

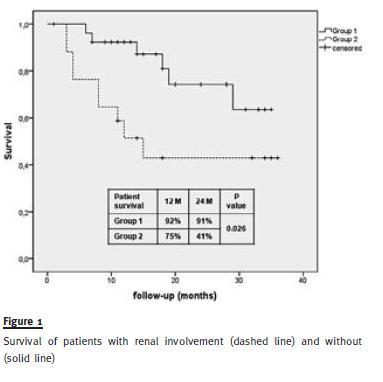
In the survival curves (Fig. 1), we found that patients with renal disease had a significantly lower survival than patients without renal disease at 12 and 24 months (75% versus 92% and 41% versus 91%, p = 0.026).
In the multivariate analysis, renal dysfunction was not associated with lower survival. We found that albumin < 3.5g/dL (HR 6.68; CI: 1.27-33.05; p= 0.025) and light chain MM (HR 7.34; CI: 1.63-49.4; p = 0.009) were associated with lower survival (Table VI).
DISCUSSION
Renal failure is a common complication of MM, but estimates of its incidence at presentation of MM depend strongly upon the definition of renal failure.
In two large series, 43% of 998 patients had a plasma creatinine concentration above 1.5 mg/dL8 and 22% of 423 patients had a plasma creatinine concentration ≥ 2.0 mg/dL14. The pathology is very heterogeneous and may involve a variety of different mechanisms.
Renal failure occurs in 25% to 75% of patients with MM and is usually the result of monoclonal immunoglobulin light chains15,16. However, in rare occasions, monoclonal heavy chains or the entire immunoglobulins may be involved. Additional factors that may contribute to renal dysfunction include dehydration, hypercalcaemia, hyperuricaemia, hyperviscosity, infection and the use of nephrotoxic drugs.
In our study, 44% of the renal involvement cases were due to cast nephropathy, which is consistent with the prevalence described in the literature8,15,16. In six patients, hypercalcaemia, hypotension and use of non-steroidal anti-inflammatory drugs were identified as the precipitants of the acute kidney injury. Glomerular injury was found in four patients.
The presence of renal disease is of prognostic importance because it is associated with a significant increase in morbidity and mortality12,17. There is a general correlation between the presence and severity of renal disease and patient survival. The mean survival in patients with myeloma is approximately 36 months, with a 5-year survival of 18 to 27%12,18,19, and renal failure is one of the most common causes of death. As a result of the poor prognosis and increased morbidity associated with renal disease, early and aggressive management of renal insufficiency and myeloma is critical. The response of the renal disease to therapy also appears to have prognostic value14.
In our population, the mortality rate was 34% (15/44), with more than one third (six patients) of all deaths being attributed to infectious complications.
Of these patients, nine had renal disease (60%), but no deaths were related to it, which is probably explained by the early detection of renal disease and its early and aggressive management.
Three patients died due to severe infections (two of pulmonary sepsis and one of sepsis related with dialysis central venous catheter), one patient died due to acute myocardial infarction, three due to haematologic progression and two patients with unknown causes (probably an arrhythmia due to AV conduction changes, as they both had cardiac amyloidosis).
In our population, patients with renal disease at presentation had lower survival than patients with preserved renal function, the majority of patients having died by 2 years after their presentation with acute kidney injury. However, in the multivariate analysis, renal involvement was not an independent predictive risk factor of mortality. This fact suggests that patients with impaired renal function have simultaneously other markers of poor prognosis. In fact, we found that those patients were at more advanced stages of disease, had higher tumour loads and had lower values of haemoglobin and albumin. This is consistent with other studies that showed that impaired renal function was a marker of poor prognosis in the unadjusted analysis17, but it was not after adjustment for tumour burden20.
This also concurs with the description of the International Scoring System for MM that identified raised creatinine (> 2 mg/dL) as a weak discriminator of prognosis in a multiple regression analysis, as most patients with renal failure have stage 3 disease13.
A univariate analysis of a series of 1027 patients with MM seen at the Mayo Clinic between 1985 and 199812, found that the following were adverse prognostic risk factors for survival: Eastern Cooperative Oncology Group (ECOG) performance status 3 or 4, serum albumin < 3 g/dL, serum creatinine ≥ 2 mg/dL, platelet count < 150,000/microL, age ≥ 70 years, beta-2-microglobulin > 4 mg/L, plasma cell labeling index ≥ 1%, serum calcium ≥ 11 mg/dL, haemoglobin < 10 g/dL and bone marrow plasma cell percentage ≥ 50%.
In our analysis, we found that low serum albumin (< 3.5g/dL) was significantly associated with mortality in the multivariate analysis. This is also consistent with an analysis of a larger population with MM13, and it is useful to demonstrate that it still carries a prognostic value in the population with severe acute kidney injury. We also found that light chain myeloma was an independent risk factor of death.
This finding is being inconsistently found in other studies in the literature. In three large reviews, light chain myeloma was reported to be not associated with a difference in prognosis12, or a significant reduction in survival13. However, other work showed that a significant reduction in survival occurs if light chain myeloma is accompanied by renal impairment at presentation21.
Therapy and treatment strategy of MM have largely changed in recent years22-24. At same time, it is becoming more important to control the disease in a long-term fashion, maintaining quality of life of patients because it is still difficult to cure the disease.
Autologous stem cell transplantation is the treatment of choice if the patient is fit enough25, but this generally requires high-dose melphalan treatment (the risks of which increase significantly in patients with impaired renal function), so patients with severe renal failure are unlikely to receive such treatments. Until recently, no chemotherapy regimen had been shown to improve survival in patients with myeloma if they were not candidates for transplantation. However, new agents (i.e., bortezomib and thalidomide) are now available which have shown survival benefit in such patients26,27.
Bortezomib, thalidomide and lenalidomide have moved to the forefront of MM treatment and have been assessed in patients with renal impairment27,28.
A substantial amount of evidence supports the use of bortezomib in this population. This agent can be administered at the full dose and schedule, regardless of the level of impairment, and has been shown to improve renal function. Lenalidomide in combination with dexamethasone has demonstrated efficacy in patients with MM who have mild to moderate renal insufficiency. In patients with severe renal impairment, this agent must be dose-adjusted according to renal function to maintain an acceptable toxicity profile.
Thalidomide may also be effective but data are limited and caution should be taken when initiating this drug in patients with renal dysfunction. Although the evidence evaluating the effectiveness of plasmapheresis in patients with acute kidney injury due to myeloma MM is limited and conflicting, some groups suggest the use of plasmapheresis for the removal of the toxic circulating light chains in those patients who have a course consistent with myeloma cast nephropathy and monoclonal free light chains in the serum or urine, or who have cast nephropathy on kidney biopsy29-31. In our population, no patient did plasmapheresis and we could not study the haematological response to chemotherapy because of the heterogeneity of therapies. However, we hope that we can do it in the future, now that therapies are being more homogeneous and treatment protocols are being defined in our hospital.
In conclusion, renal impairment is a common and severe complication of MM. It seems that nephrologists are of great importance and significant collaborators of haematologists in the diagnosis and treatment of MM, since renal manifestations are commonly detected first. Since patients with renal involvement seem to have poor prognosis, it is of crucial importance to have high suspicion index to do an early diagnosis and to promote an early and aggressive management of renal insufficiency and myeloma. In recent years, introduction of novel agents has changed treatment strategies and has offered an opportunity to patients with severe renal impairment to be treated. The increase in the number of treatment options means that personalized medicine which selects a treatment corresponding to the systemic condition of the patient, and the purpose of the treatment will be of great importance. Also we should consider how we could help patients through the treatment to live long actively in the society.
References:
1. RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004;351(18):1860–1873
2. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000;50(1):7-33 [ Links ]
3. Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol 2004.127(3):299–304 [ Links ]
4. Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55(1):10–30 [ Links ]
5. Rajkumar SV, Kyle RA. Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc 2005;80(10):1371–1382 [ Links ]
6. International Myeloma Working Group: Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br J Haematol 2003;121(5):749–757 [ Links ]
7. Clark AD, Shetty A, Soutar R. Renal failure and multiple myeloma: Pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev 1999;13(2):79–90 [ Links ]
8. Winearls CG. Acute myeloma kidney. Kidney Int 1995;48(4):1347–1361 [ Links ]
9. Hutchison CA, Batuman V, Behrens J, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 2011;8(1):43-51 [ Links ]
10. Ludwig H, Bolejack V, Crowley J, et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol 2010;28(9):1599-1605 [ Links ]
11. Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: Reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol 2000;65(3):175–181 [ Links ]
12. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78(1):21-33 [ Links ]
13. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23(15):3412–3420 [ Links ]
14. Blade J, Fernandez-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998;158(17):1889-1893 [ Links ]
15. Nasr SH, Valeri AM, Sethi S, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis 2012;59(6):786-794 [ Links ]
16. Montseny JJ, Kleinknecht D, Meyrier A, et al. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant 1998;13(6):1438-1445 [ Links ]
17. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council Trials Between 1980 and 2002Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005;23(36):9219–9226 [ Links ]
18. Irish AB, Winearls CG, Littlewood T. Presentation and survival of patients with severe renal failure and myeloma. QJM 1997;90(12):773–780. [ Links ]
19. Rayner HC, Haynes AP, Thompson JR, Russell N, Fletcher J. Perspectives in multiple myeloma: Survival, prognostic factors and disease complications in a single center between 1975 and 1988. Q J Med 1991;79(290):517–525 [ Links ]
20. Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 2007;48(2):337–341 [ Links ]
21. Drayson M, Begum G, Basu S, et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials. Blood 2006;108(6):2013-2019 [ Links ]
22. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111(5):2516–2520 [ Links ]
23. Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol 2012;87(1):78-88 [ Links ]
24. Bird JM, Owen RG, DSa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011;154(1):32-75 [ Links ]
25. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003;348(19):1875–1883 [ Links ]
26. Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007;370(9594):1209–1218 [ Links ]
27. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359(9):906–917 [ Links ]
28. Suzuki K. Diagnosis and treatment of multiple myeloma and AL amyloidosis with focus on improvement of renal lesion. Clin Exp Nephrol 2012;16(5):659–671 [ Links ]
29. Cserti C, Haspel R, Stowell C, Dzik W. Light-chain removal by plasmapheresis in myeloma-associated renal failure. Transfusion 2007;47(3):511–514 [ Links ]
30. Pillon L, Sweeting RS, Arora A, et al. Approach to acute renal failure in biosy proven myeloma cast nephropathy: is there still a role for plasmapheresis? Kidney Int 2008;74(7):956-961 [ Links ]
31. lark WF, Stewart AK, Rock GA, et al. Canadian Apheresis Group. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143(11):777-784. [ Links ]
Dra Clara Santos
Nephrology Department
Centro Hospitalar Vila Nova de Gaia
Rua Conceicao Fernandes, Vilar de Andorinho 4430-502
Vila Nova de Gaia, Portugal
E-mail: mclaracsantos@gmail.com
Conflict of interest statement: None declared.
Received for publication: 20/07/2013
Accepted in revised form: 05/11/2013














