Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.4 Lisboa dez. 2013
CASE REPORT
Renal sarcoidosis presenting as renal failure: report of three cases with interstitial nephritis and normocalcemia
Insuficiência renal como manifestação inaugural de sarcoidose: relato de três casos de nefrite intersticial e normocalcemia
Margarida Fonseca1, Francisca Barros2, Raquel Vaz2, Ana Nunes2, Ana Cerqueira2, Augusta Praca2, Susana Sampaio2
1Department of Internal Medicine, Hospital de Braga. Braga, Portugal.
2Department of Nephrology, Centro Hospitalar de São João. Porto, Portugal.
ABSTRACT
Sarcoidosis is a chronic inflammatory disorder of unknown cause. The histopathological feature is the presence of the non-caseating granuloma in any organ involved. The mechanisms most frequently implicated in renal dysfunction are due to disturbances in calcium metabolism; more rarely, granulomatous interstitial nephritis and glomerular disease may occur. The authors report three cases of sarcoidosis presented as interstitial nephritis with renal failure. Prednisolone treatment was performed at a dose of 1 mg/kg/day with partial and sustained recovery of renal function.
Key words: Acute kidney injury; granulomatous interstitial nephritis; prednisolone; sarcoidosis.
RESUMO
A sarcoidose e uma doença inflamatória cronica, de atingimento multissistemico, cuja etiologia permanece ainda por identificar. Caracteriza-se, do ponto de vista histopatológico, pela formação de granulomas não caseosos em qualquer órgão envolvido. Os mecanismos mais frequentemente implicados na disfunção renal devem-se aos distúrbios no metabolismo do cálcio; mais raramente podem ocorrer nefrite intersticial granulomatosa e doença glomerular. Os autores apresentam três casos de nefrite intersticial com o achado de insuficiência renal como manifestação inaugural de sarcoidose. Foi efectuado tratamento com prednisolona na dose de 1 mg/Kg/dia, verificando-se recuperação parcial e sustentada da função renal.
Palavras-chave: Lesao renal aguda; nefrite intersticial granulomatosa; prednisolona; sarcoidose.
INTRODUCTION
Sarcoidosis is a chronic inflammatory disorder of unknown cause that is characterized by its pathological hallmark, the non-caseating granuloma1. The respiratory tract and the liver are predominantly affected. However, any organ can be involved. Clinically relevant renal involvement of sarcoidosis is rare because most patients with renal manifestation remain asymptomatic2. Although this disorder may involve the kidney in several ways, the precise prevalence of renal involvement is not known3. Renal dysfunction in sarcoidosis may occur due to a number of causes. The most common cause of renal involvement is hypercalcemia which is related to dysregulated production of 1, 25-dihydroxyvitamin D3 by activated macrophages trapped in pulmonary alveoli and granulomas inflammation, leading to nephrocalcinosis, nephrolithiasis and renal dysfunction4-6. In addition, granulomatous interstitial nephritis (GIN), has been noticed in approximately one third of adult patients with renal sarcoid7,8. Glomerulonephritis or renal artery stenosis secondary to sarcoid vasculopathy are rare manifestations of sarcoidosis8. We report three cases of sarcoidosis presented as interstitial nephritis with renal failure. Prednisolone treatment was performed with partial but sustained recovery of renal function.
CASE REPORTS
Case 1
A 25-year-old white female without relevant previous diseases was referred to our hospital presenting anorexia, vomiting, asthenia, fever and unintentional weight loss of 10 kilograms lasting two months. She recurred multiple times to different emergency services during that period, always getting discharged without a definite diagnosis.
Her physical examination was irrelevant. Initial laboratory tests revealed serum creatinine (SCr) 1.76 mg/dL, blood urea nitrogen (BUN) 34 mg/dL, haemoglobin (Hb) 10.4 g/dL, white blood cell (WBC) count 2.990/uL, eosinophils 620/uL, γ GT 501 U/L, alkaline phosphatase (AF) 357 U/L and serum (albumin corrected) calcium 9.9 mg/dL. Urinalysis showed mild proteinuria (0.7 g in 24-hour protein measurement), mild haematuria and no dysmorphic red blood cells or casts.
Abdominal and renal ultrasound showed an enlarged liver with regular borders and scattered, slightly increased areas of echogenicity as well as enlarged kidney size with normal echogenicity, without calculi or hydronephrosis. Chest X-ray showed mild diffuse interstitial disease without hilar lymphadenopathy and complementary thoraco-abdominalpelvic computarized tomography (CT) scan revealed diffuse pulmonary micronodules, hepatosplenomegaly, enlarged kidney and multiple retroperitoneal lymphadenopathies. Further laboratory tests were normal for protein electrophoresis, immunology screening, culture and viral markers, but showed elevated serum angiotensin converting enzyme (ACE) levels (Table I).
A kidney biopsy was performed 10 days after ward admission while creatininaemia was 2.14mg/dL, revealing nine glomeruli in light microscopy, extensive tubulointerstitial infiltration with lymphocytes and multinucleated giant cells forming interstitial non-necrotizing granulomata (Figure 1 and Figure 2). These findings suggested a chronic granulomatous tubulointerstitial nephritis, consistent with stage II systemic sarcoidosis. The patient started oral prednisolone (1 mg/kg/day) and, one week later, complete renal function recovery was accomplished (SCr 1.0mg/dL). Treatment was continued for two months, then tapered off slowly. One year later, the patient maintains prednisolone at 5mg/day without further clinical symptoms and SCr 1.3 mg/dL.
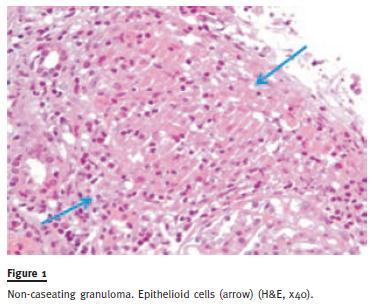
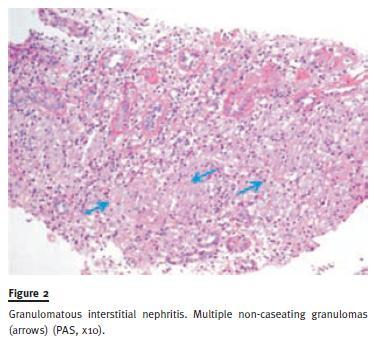
Case 2
A 29-year-old white male with declining renal function was referred to our nephrology outpatient clinic.
His medical history was remarkable for recurrent renal colic but concurrent non-steroidal anti-inflammatory drug abuse was denied. He reported asthenia, anorexia, generalized myalgia and oedema of both lower limbs lasting one month.
His physical examination was remarkable only for bilateral malleolar oedema. Initial laboratory showed BUN 23.8 mg/dL, SCr 1.84 mg/dL and serum (albumin-corrected) calcium 10.2 mg/dL. Urinary sediment had no cells or casts; 24-hour urine collection showed 0.42 g of protein and urinary calcium of 0.08 g/day.
Renal ultrasound revealed normal sized kidneys with increased echogenicity and decreased differentiation but no evidence of obstruction or nephrolithiasis.
Additional laboratory tests revealed mild normocytic normochromic anaemia and an elevated serum ACE level (Table I).
A kidney biopsy was performed three months later, with SCr 2.6 mg/dL, and revealed a similar picture of extensive tubulointerstitial nephritis, interstitial fibrosis and tubular atrophy, as in the previous patient, with extensive epithelioid granulomatous lesions without necrosis (Figure 3 and Figure 4). Special stains for acid-fast organisms were negative. At this time, the patient complained about dyspnoea on strenuous efforts. Pulmonary function tests were negative. Chest X-ray showed bilateral diffuse alveolar-interstitial infiltration, with bilateral hilar lymph nodes. Endobronchial fibroscopy gathered a bronchoalveolar lavage (BAL) fluid with intense lymphocytosis with a predominance of CD4 and CD4/CD8 ratio of 8. Needle aspiration of a mediastinal lymph revealed non-caseating epithelioid cell granuloma.
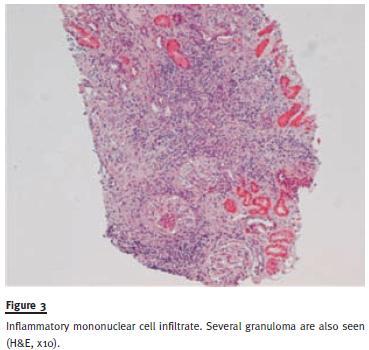
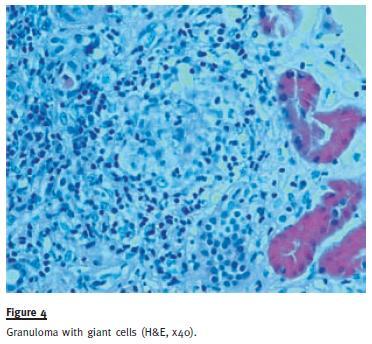
A systemic sarcoidosis diagnosis was established and the patient was treated with three pulses of 500 mg of intravenous methylprednisolone for a total of three days and continued on 1mg/kg/day prednisolone scheme for two months, with improvement of both renal function and respiratory symptoms.
Two years later, while on a 5 mg/day prednisolone daily dose, SCr 1.4 mg/dL is maintained.
Case 3
A 48-year-old white male with 3 years of hyperuricaemia and gouty arthritis was referred to our nephrology outpatient consultation due to a newly diagnosed anaemia and declining renal function. At the appointment, he further reported systemic symptoms including asthenia, anorexia and muscular weakness on the previous 4 months. He was taking alopurinol 300mg and, six months previously, he had a normal serum creatinine on routine evaluation.
Physical examination was unremarkable. Initial laboratory tests revealed BUN 31.8 mg/dL, SCr 2.78 mg/dL, serum (albumin-corrected) calcium 9.9 mg/dL. Urinalysis showed no active sediments. A 24-hour urine collection showed 0.2 g of protein and urinary calcium excretion was normal.
Renal ultrasound showed normal kidneys. Chest x-ray revealed bilateral hilar prominence and a nodular lesion of about 9 mm. High-resolution CT-scan further illustrated mediastinal and hilar lymph nodes and nodular densification in lung parenchyma. Further blood analysis was normal for protein electrophoresis, viral serologic markers, immunology screening and bacteriologic cultures (Table I). Serum ACE levels were normal (< 3 U/L).
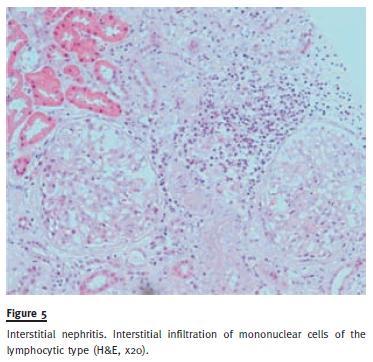
Urine was negative for alcohol-acid fast bacilli and culture and there was no detectable Bence Jones protein. BLA revealed lymphocytic alveolitis and normal CD4/CD8 ratio (1:1). Malignant cells were also absent. Histopathological examination of subcarinal lymph node revealed aggregates of epithelioid macrophages with sketch of epithelioid granulomas without necrosis or giant cells. Cytology was negative for malignant cells. Renal biopsy specimen revealed large clusters of precipitated calcium in the medullary region. Interstitial fibrosis was present in about 20% of the fragment. Tubular atrophy with hyaline casts was seen and a mononuclear cell infiltration was found, mostly of the lymphocytic type (Fig. 5).
The diagnosis of stage II systemic sarcoidosis with pulmonary and renal involvement was finally established.
Treatment with prednisolone, 1 mg/kg/day, was initiated and one month later renal function showed some improvement, with SCr 2.17mg/dL.
Corticosteroid therapy was gradually tapered without relapse and was discontinued six months later. Serum creatinine reached a minimum of 1.81mg/dL. After one year without treatment, the patients SCr is of 2mg/dL.
DISCUSSION
Sarcoidosis is a systemic disease of unknown aetiology, characterized by chronic non-caseating granulomatous inflammation with tissue destruction7.
Diagnosis of sarcoidosis relies on the demonstration of non-caseating granulomas in a biopsy specimen and clinical exclusion of other causes of granulomatous inflammation. Renal involvement is only seen in a small number of patients. The incidence of renal sarcoidosis varies widely (0.7%–10%), in part due to varying definitions used in different studies9,10. Although sarcoidosis is primarily a granulomatous inflammation, granulomatous interstitial nephritis (GIN) accounts for only a small proportion of clinically significant kidney failure in patients with sarcoidosis. The mechanism most frequently implicated in renal dysfunction is due to disturbances in calcium metabolism. Macrophages within sarcoid granulomas have elevated 1α-hydroxy lase activity resulting in overproduction of 1, 25-dihydroxyvitamin D3 which in turn leads to hypercalcaemia, nephrocalcinosis or urolithiasis11.
In sarcoid nephropathy, hypercalcaemia occurs in approximately 10% of patients, whereas hypercalciuria is about three times more frequent12.
Renal function impairment without calcium nephropathy is uncommon. In all three cases presented here, serum calcium levels were normal. No renal calculi were found. After excluding abnormalities affecting calcium homeostasis, tubulointerstitial diseases are the most commonly encountered renal abnormalities in sarcoidosis – they are histopathologically described as GIN13. True prevalence of GIN in sarcoidosis is unknown, mainly because a systematic kidney biopsy is rarely performed, even with mild renal signs, and may be present without pulmonary involvement14. Also, one should remember that GIN by itself is not pathognomonic of sarcoidosis. Granulomatous interstitial nephritis has been associated with medication, infections, crystal deposits, paraproteinaemia, and Wegeners granulomatosis also is seen in an idiopathic form15,16.
Other causes of GIN have to be ruled out, and notably drug induced. Medicines implicated include anticonvulsants, antibiotics, non-steroidal anti-inflammatory drugs, allopurinol and diuretics, although proof that a particular drug is responsible is often circumstantial15. Supportive evidence may include a temporal relation between starting the drug and the development of renal failure or cessation of the drug and an improvement in renal function. In two of our patients (cases 1 and 2) abnormal renal function was noted at a time when no medication was taken. In the other patient (case 3) there was no clear relation between starting a drug and the development of disease, since he had been medicated with allopurinol three years before the diagnosis of renal failure.
Moreover, in our series of cases, two patients (cases 2 and 3) had the finding of granulomas in another organ, which turns the diagnosis of drug-induced GIN unlikely. Consistent with a pattern of tubulointerstitial disease, proteinuria is either absent or mild.
Urine analysis shows leucocytes and granular casts. Rarely, the patient may present as frank haematuria lasting several weeks. The GIN is usually associated with enlarged kidneys mimicking renal amyloidosis or diabetic nephropathy. In the first patient described here, abdominal ultrasound showed an enlarged liver with scattered, slightly increased areas of echogenicity.
This suggested sarcoidosis of the liver, although liver biopsy was not performed. Serum ACE concentration is a poor marker of active renal lesion and may even be normal in active GIN with severe renal failure12,17, as we noticed in the third patient.
In most cases, diagnosis of GIN is made in the context of typical extra-renal manifestations of sarcoidosis and/or hypercalcaemia. Rarely, renal involvement may be isolated and preceding other sites of the disease for months to years18. In all patients presented here, occurrence of renal failure led to the diagnosis of sarcoidosis. Glomerular involvement in sarcoidosis is not very common and the exact mechanisms of glomerular disease in sarcoidosis are not known. The spectrum of commonly reported glomerular diseases include focal segmental sclerosis, membranous glomerulonephritis (GN), mesangioproliferative glomerulonephritis, mesangiocapillary glomerulonephritis, IgA nephropathy and crescentric glomerulonephritis.
Due to the absence of a consistent glomerular pathology and a well described aetiological pathway, most cases are believed to be coincidental associations12,19.
Many of the agents used in the therapy of sarcoidosis target, among other cytokines, TNF-alpha.
These agents include non-specific inhibitors of TNFalpha release, such as steroids, methotrexate, azathioprine, antimalarials, and phosphodiesterase inhibitors, as pentoxifylline and thalidomide. The mainstay of treatment of sarcoid GIN is glucocorticoids.
The other agents have been tried in systemic extra-renal sarcoidosis although no data on treating renal sarcoidosis exists12,20,21. Initial treatment of GIN requires a daily dose of prednisone or prednisolone preferably 1 – 1.5 mg/kg. Response to treatment can often be dramatic in terms of improvement of renal insufficiency. An important point to realize here is that steroid treatment has to be prolonged and must exceed at least six months as nephropathy relapses are very frequent with short term therapy.
A commonly followed strategy is to give the initial dose for two months followed by progressive taper and switching to an alternate day therapy. A maintenance therapy period for one year at least is recommended15,22.
One of our patients (case 2) was started on steroid therapy with 3 pulses of intravenous methylprednisolone. The authors are unaware of the exact reason why methylprednisolone was considered. We presume that this was probably because sarcoidosis is often mixed up with autoimmune diseases. There is no consensus on the appropriate therapy for patients who are intolerant of corticosteroids or who relapse frequently upon dose reduction or discontinuation. The use of alternative agents may be indicated if the side effects of corticosteroids are intolerable or if there is progression of disease despite adequate therapy. Three agents are currently available as specific TNF- α antagonists: etanercept, infliximab and adalimumab23. Etanercept is an ineffective therapeutic agent in the treatment of sarcoidosis24,25. In contrast, multiple case reports and a small randomized controlled trial26 suggest that infliximab is an effective therapy in both refractory pulmonary sarcoidosis and extrapulmonary diseases, including uveitis, neurosarcoidosis, cardiac sarcoidosis and GIN. There are two case reports of treatment with infliximab in renal sarcoidosis. Thumfart and colleagues8 described the case of a boy presenting with severe arterial hypertension and acute renal failure caused by an isolated sarcoid granulomatous interstitial nephritis. Renal function improved initially with prednisone treatment but later the patient showed signs of severe steroid toxicity and progressive renal failure. Monthly treatment with infliximab was started, resulting in steady improvement in renal function and resolution of renal granuloma.
In addition, antihypertensive medication could be reduced. Ahmed and associates27 presented a patient with acute renal failure due to isolated granulomatous infiltration of the renal parenchyma. Renal biopsy showed granulomatous interstitial nephritis with non-caseating granulomas. There was no evidence of extrarenal sarcoid involvement. Prednisone 60 mg daily resulted in significant improvement in renal function. Because of recurrent flares on steroid taper and steroid toxicity, treatment with infliximab was instituted and resulted in stabilization of renal function. Gupta et al.28 described the successful treatment of a case of granulomatous interstitial nephritis using adalimumab. The combination of clinical improvement and complete lack of granulomatous features on follow-up biopsy strongly suggests immunomodulation by adalimumab-induced resolution of sarcoid nephropathy. Adalimumab, like infliximab, is not without systemic side effects although most are relatively minor. Among those causing the most concern is the risk of reactivation of tuberculosis. In the cohort described by Preddie et al.29, eight patients had received corticosteroids and had experienced relapses, intolerance and resistance to therapy. All of them were treated with mycophenolate mofetil (MMF) for interstitial nephritis. In each case, serum creatinine stabilized or improved on therapy. Five patients had been able to discontinue treatment with MMF successfully. The remaining three patients were maintained on MMF, unable to discontinue without relapse. This may mean that there was a trading of steroid dependence and adverse effects for MMF dependence and adverse effects. Therefore, optimal therapeutic management of GIN can only be determined after further investigation.
Our three patients presented renal failure as the first evidence of sarcoidosis. All of them had also some degree of pulmonary involvement at the moment of diagnosis despite the absence of respiratory symptoms.
The first two patients had renal failure secondary to GIN. The last patient had only evidence of interstitial nephritis and deposits of medullary calcium in his renal biopsy; nevertheless, we cannot rule out the hypothesis of concurrent GIN; even though we had not found granulomatous lesions, it is possible that these had not been seen because of randomness of biopsy sampling. Other causes of granulomatous lesions in all three patients had been excluded.
According to some reports7,12, renal sarcoidosis should be considered a potentially serious manifestation of the disease, with rapidly progression of renal failure to end stage renal disease despite treatment with corticosteroids. All our patients had been treated with prednisolone at the time of diagnosis, with satisfactory recovery of their renal function. This is probably explained by an aggressive treatment with steroids since the beginning.
In conclusion, we have presented three patients with interstitial nephritis with renal failure as the presenting manifestation of sarcoidosis. All patients were started on a forward regimen of 1 mg/Kg/day of prednisolone, with renal function improvement.
References
1. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336(17):1224-1234. [ Links ]
2. Mayer C, Muller A, Halbritter J, Wirtz H, Stumvoll M. Isolated renal relapse of sarcoidosis under low-dose glucocorticoid therapy. J Gen Intern Med 2008;23(6):879-882. [ Links ]
3. Ohashi N, Yonemura K, Hirano M, et al. A patient with sarcoidosis presenting with acute renal failure: Implication for granulomatous interstitial nephritis and hypercalcemia. Intern Med. 2002;41(12):1171-1174. [ Links ]
4. Romer FK. Renal manifestations and abnormal calcium metabolism in sarcoidosis. Q J Med 1980;49(195):233–247. [ Links ]
5. Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003;361(9363):1111-1118. [ Links ]
6. Ikeda A, Nagai S, Kitaichi M, et al. Sarcoidosis with granulomatous interstitial nephritis: report of three cases. Intern Med 2001;40(3):241-245. [ Links ]
7. Robson MG, Banerjee D, Hopster D, Cairns HS. Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 2003;18(2):280–284. [ Links ]
8. Thumfart J, Muller D, Rudolph B, Zimmering M, Querfeld U, Haffner D. Isolated sarcoid granulomatous interstitial nephritis responding to infliximab therapy. Am J Kidney Dis 2005;45(2):411-414. [ Links ]
9. Shah R, Shidham G, Agarwal A, Albawardi A, Nadasdy T. Diagnostic utility of kidney biopsy in patients with sarcoidosis and acute kidney injury. Int J Nephrol Renovasc Dis 2011;4:131–136. [ Links ]
10. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologists perspective. Am J Kidney Dis 2006;48(5):856–870. [ Links ]
11. Inui N, Murayama A, Sasaki S, et al. Correlation between 25-hydroxy-vitamin D3 1αhydroxylase gene expression in alveolar macrophages and the activity of sarcoidosis. Am J Med 2001;110(9):687-693. [ Links ]
12. Tsiouris N, Kovacs B, Daskal II, Brent LH, Samuels A. End-stage renal disease in sarcoidosis of the kidney. Am J Kidney Dis 1999;34(5):7-11. [ Links ]
13. Sheffield EA. Pathology of sarcoidosis. Clin Chest Med. 1997;18(4):741-754. [ Links ]
14. Miyoshi K, Okura T, Manabe S, Watanabe S, Fukuoka T, Higaki J. Granulomatous interstitial nephritis due to isolated renal sarcoidosis. Clin Exp Nephrol. 2004;8(3):279–282. [ Links ]
15. Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol 2007;2(2):222-230. [ Links ]
16. Monge M, Miquel O, Dugardin F, t al. Sarcoidosis and the kidney: not only the granulomatous interstitial nephritis. Clin Nephrol 2009;71(2):192-195.
17. Hannedouche T, Grateau G, Noel H, t al. Renal granulomatous sarcoidosis: report of six cases. Nephrol Dial Transplant 1990;5(1):18-24.
18. Mehta T, Ganguli A, Haji-Momenian M. Sarcoidosis and Kidney Disease.In: Chronic Kidney Disease, Prof. Monika GoYz (Ed.).In Tech.Rijeka(Croatia),, 2012;87-106 [ Links ]
19. Muther RS, McCarron DA, Bennett WM. Renal manifestations of sarcoidosis. Arch Intern Med 1981;141(5):643-645. [ Links ]
20. Baughman RP, Lynch JP. Difficult treatment issues in sarcoidosis. J Intern Med 2003;253(1):41-45. [ Links ]
21. Agrawal V, Crisi G, DAgati VD, Freda BJ. Renal sarcoidosis presenting as acute kidney injury with granulomatous interstitial nephritis and vasculitis. Am J Kidney Dis 2012;59(2):303-308. [ Links ]
22. Guenel J, Chevet D. Interstitial nephropathies in sarcoidosis. Effect of corticosteroid therapy and long-term evolution. Retrospective study of 22 cases. Nephrologie 1988;9(6):253-257. [ Links ]
23. Callejas-Rubio JL, Lopez-Perez L, Ortego-Centeno N. Tumor necrosis factor-alpha inhibitor treatment for sarcoidosis. Ther Clin Risk Manag 2008;4(6):1305-1313. [ Links ]
24. Ulz JP, Limper AH, Kalra S, t al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124(1):177-185.
25. Baughman RP, Lower EE, Bradley DA, Raymond LA, Kaufman A. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest 2005;128(2):1062-1067. [ Links ]
26. Baughman RP, Drent M, Kavuru M, t al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174(7):795-802.
27. Ahmed MM, Mubashir E, Dossabhoy NR. Isolated renal sarcoidosis: a rare presentation of a rare disease treated with infliximab. Clin Rheumatol 2007;26(8):1346-1349. [ Links ]
28. Gupta R, Beaudet L, Moore J et al. Treatment of sarcoid granulomatous interstitial nephritis with adalimumab. NDT Plus. 2008; 2(2):139-142. [ Links ]
29. Preddie DC, Markowitz GS, Radhakrishnan J, et al. Mycophenolate mofetil for the treatment of interstitial nephritis. Clin J Am Soc Nephrol 2006;1(4):718-722. [ Links ]
Dra Margarida Fonseca
Department of Internal Medicine
Hospital de Braga, Sete Fontes – Sao Vitor
4710-243 Braga, Portugal
E-mail: magutas@gmail.com
Conflict of interest statement: None declared
Received for publication: 18/08/2013
Accepted in revised form: 25/11/2013














