Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.1 Lisboa mar. 2014
CASE REPORT
Mineral and Bone Disease (MBD)
Rita Birne1, Teresa Adragão1, Aníbal Ferreira2, Jorge Dickson1, Rui Silva3, Ana Casqueiro1, Regina Oliveira1, Ana Rita Martins1, João Torres1, Patrícia Matias1, Patrícia Branco1, Cristina Jorge1, André Weigert1, Margarida Bruges1, Domingos Machado1
1 António Pina Transplant Unit, Department of Nephrology, Hospital de Santa Cruz, CHLO. Lisboa, Portugal.
2 Kidney Transplant Unit, Department of Nephrology, Hospital Curry Cabral,CHLC. Lisboa, Portugal.
3 Nephrology Department, Hospital Espírito Santo. Évora, Portugal.
ABSTRACT
A 50-year-old post-menopausal recipient of a kidney allograft with bone pain, osteoporosis, persistent hypercalcaemia and elevated parathormone (PTH) levels, despite a satisfactory graft function, was treated with bisphosphonates and cinacalcet starting, respectively, 5 and 6 months after renal transplantation (RT).
Sixteen months after treatment, there was improvement of bone mineral density (BMD) measured by dualenergy X-ray absorptiometry (DEXA). A bone biopsy was taken, unveiling a surprising and worrisome result.
Post-RT bone disease is different from classic CKD-MBD and should be managed distinctly, including, in some difficult cases, an invasive evaluation through the performance of a bone biopsy, as suggested in the KDIGO guidelines.
Key Words: Bone biopsy; cinacalcet; ckd-mbd; hypercalcaemia; kidney transplantation; osteoporosis.
RESUMO
Uma transplantada renal de 50 anos, pós menopáusica, com dor óssea, osteoporose, hipercalcémia persistente e níveis elevados de paratormona, apesar de uma razoável função do enxerto renal, foi tratada com bifosfonatos e cinacalcet com início 5 e 6 meses, respectivamente, após a transplantação renal. Verificouse melhoria da densidade óssea medida por DEXA. Foi realizada uma biópsia óssea que revelou um resultado inesperado e preocupante. A doença óssea após a transplantação renal é diferente da DMO-DRC clássica e deve ser gerida de forma distinta. Nalguns casos, como o ilustrado na presente apresentação, justifica-se o diagnóstico invasivo através do recurso à realização de uma biópsia óssea, como é proposto nas orientações da KDIGO.
Palavras-Chave: Biópsia óssea; cinacalcet; dmo-drc; hipercalcemia; osteoporose; transplantação renal.
INTRODUCTION
The most frequent post-renal transplantation (RT) bone disease is characterized by accelerated loss of bone mineral density and increased risk of fractures.
There are no randomized clinical trials in kidney transplant patients examining bone specific therapies on clinical outcomes, such as fractures or cardiovascular disease events. Bone disease in kidney transplant patients is heterogeneous, involving pre-existing renal osteodystrophy, the effects of immunosuppression therapy on bone and the effects of reduced renal function after RT; these factors overlap and result in different pathologic states. Several pharmacologic therapies that target multiple components of the MBD imbalance have been used.
Kidney Disease: Improving Global Outcomes (KDIGO) clinical practical guidelines for the care of kidney transplant recipients includes the management of transplant bone disease. The KDIGO latest guidelines on CKD-MBD were issued in 2009; a conference on controversies on this very issue was announced to be held in October 2013. Its purpose was to evaluate what is known about CKD-MBD, what can be done with what is known and what needs to be learned1.
This case report of hypercalcaemic hyperparathyroidism after RT discloses that urgent need.
CASE REPORT
A 50-year-old white woman was submitted to RT in October 2009. Her immunosuppression consisted of basiliximab (first and fourth RT days), methylprednisolone (500-250-125-80 mg in RT days 1-4, respectively), prednisone (35 mg at RT day 5, followed by progressive tapering until 5 mg at RT day 30), tacrolimus and mycophenolate mofetil. She had autosomal dominant polycystic kidney disease and had been on haemodialysis since January 2005. Prior to RT there was a long history of symptomatic herniated vertebral disc; she was referred to Neurosurgery, but only functional rehabilitation was recommended. She had no other relevant comorbidity.
Age of menarche was 13 years, with spontaneous menopause at age 47. She was slightly overweight (body mass index 27) and a non-smoker.
While on haemodialysis, the patient´s mineral serum values were remarkable for hyperphosphatemia, with phosphorus ranging 4.1-7.1 mg/dl, calcium 8.0-9.9 mg/dl, calcium-phosphorus product 35-61 (mg/dl)2 and intact parathormone (iPTH) between 108-170 pg/ml . She was on sevelamer 800 mg, 6 to 9 pills qd and calcium carbonate 1000 mg 3 to 6 pills qd for over three years; no vitamin D or calcimimetic were taken.
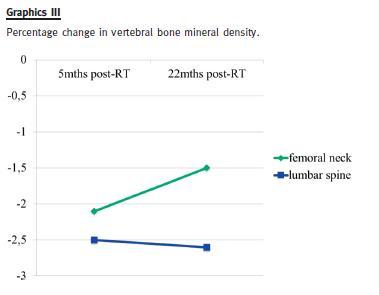
Five months after RT her gynaecologist found she had reduced BMD: a dual-energy x-ray absorptiometry (DEXA) revealed lumbar spine osteoporosis (T-score -2.5 SD) and femoral neck osteopenia (T-score -2.1 SD). He prescribed alendronic acid 70 mg every week.
Six months after RT, the patient maintained reasonable kidney graft function, with a serum creatinine of 1.1 mg/dl (estimated glomerular filtration rate by CKD-EPI Creatinine Equation of 59 ml/min/1.73m2).
She had persistent moderate hyperparathyroidism (iPTH 220 pg/ml) and hypercalcaemia (maximum 11.4 mg/dl). Cinacalcet was then initiated, 30 mg po qd.
Plasma calcium levels slightly decreased, without a significant PTH levels change (190-210 pg/ml) (Graphics I and II).
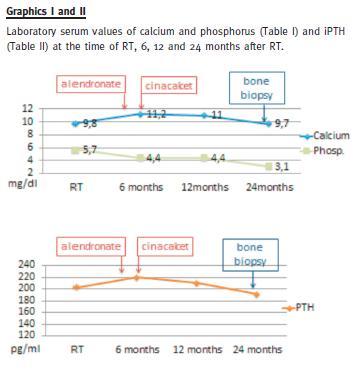
Eight months after RT she suffered a traumatic hip fracture.
Twenty-two months after RT a new DEXA-scan showed a significant increase of femoral neck bone density (T-score -1.5 SD), compared with the previous exam; lumbar spine density was similar (T-score -2.6 SD).
During all RT period, the patient continued to suffer widespread bone pain, mostly lumbar pain, which she recognized as a consequence of the degenerative bone disease identified well before RT. This clinical condition interfered markedly with her wellbeing, not only because of the psychological distress associated with constant pain, but also by the progressively compromised physical activity. Besides that, this period had been unremarkable, despite a fall that resulted in the bone fracture previously referred and some minor urinary tract infections.
Two years after RT (at the 23rd month), a bone biopsy was performed (Fig. 1). Serum PTH was still elevated at 191 pg/ml; calcium, phosphorus and alkaline phosphatase were within normal values; 25-hydroxy vitamin D level was 41.3 nmol/L (normal values 75-250 nmol/L); thyroid blood tests were normal.
The biopsy revealed severe osteoporosis and adynamic bone disease (Frozen Bone), with normal bone mineralization. The patient maintained satisfactory graft function (serum creatinine 0.9-1.2 mg/dl).
Alendronate and cinacalcet were immediately discontinued and cholecalciferol (1.334 IU of vitamin D3 per day) was added. A few weeks later, the patient felt quite better, with bone pain remission and physical activity resumed. Weight-bearing physical activity was encouraged, and she started gymnastic classes with great physical improvement. Her most recent relevant serum laboratory values, 10 months after the bone biopsy, are creatinine 1.19 mg/dl, calcium 11.1 mg/dl, phosphorus 2.5 mg/dl, PTH 139.5 pg/ml. She has a vascular calcification Adragão score of 0 /8 (no vascular calcification observed on pelvis and hand plain x-ray) and a Kauppila score of 2 /24 (vascular calcification on abdominal aorta seen on lateral abdomen plain x-ray.)
DISCUSSION
We report a case of CKD-MBD in a post-menopausal kidney transplant recipient (after almost 5 years on haemodialysis), who had lumbar spine osteoporosis and femoral osteopenia with eGFR around 60 mL/min/1.73m2, treated with bisphosphonates to whom, in face of persistent hypercalcaemia and hyperparathyroidism, cinacalcet was added. Skeletal history was significant for pre-RT symptomatic herniated vertebral disc and progressive disabling bone pain thereafter. Hypercalcaemia came to normal values and PTH remained high. Meanwhile, a (traumatic) bone fracture occurred. She maintained reasonable good graft function and bone density (by DEXA scan) was ameliorated, but her symptoms did not improve.
A bone biopsy was diagnostic for adynamic bone disease.
This case illustrates the mandatory use of bone histomorphology to manage BMD in RT patients. The risks associated with bisphosphonates and calcimimetics on RT, despite good graft function, are enlightened here; although prescribed according to prevailing recommendations, their use resulted in unwanted clinical consequences.
Low bone mineral density
Post-RT bone disease comprises a spectrum of metabolic alterations of bone remodelling that include pre-existing renal osteodystrophy alterations, as well as new factors that occur after transplantation, being influenced by immunosuppressive therapy, kidney transplant function, hypophosphatemia, and disturbances in the fibroblastic growth factor 23-PTH-vitamin D axis2,3. Many studies in transplant recipients have shown a negative correlation between glucocorticoid cumulative dose and BMD. The mechanisms whereby glucocorticoids may affect bone metabolism are multifactorial; these drugs increase osteoclastic resorption, decrease osteoblastic activity and promote osteoblast apoptosis; they also may indirectly affect bone metabolism by decreasing intestinal calcium absorption and increasing renal calcium wasting, leading to increased PTH secretion. Secondary hypogonadism further reduces bone formation3,4. The role of calcineurin inhibitors regarding rates of bone mass loss is less clear and limited information is available on mycophenolate mofetil or basiliximab. Our patient did not have a high cumulative glucocorticoid dose, although she was not on a corticoid-sparing regimen; she also had the physiologic decrease in gonadal steroids that is associated with menopause. Pre-RT osteodystrophy (with iPTH in the low/normal range), highly daily calcium carbonate over that period (3 to 6 grams), corticosteroid use, hypogonadal state, bisphosphonate and cinacalcet therapy likely contributed to her adynamic bone disease.
Accelerated loss of bone density occurs in the first year after RT, mainly in the first 3 to 6 months; after the first year, studies are conflicting5-9. The use of bisphosphonates post-transplantation has been shown to prevent loss or increase BMD10-15. However, there has generally been no evidence of fracture reduction16, and bisphosphonate treatment carries the risk of inducing or prolonging adynamic bone disease in the presence of renal dysfunction17.
Bisphosphonate deposition in the bone mineralization can last 10 years, as emphasized in a recent review by Susan Ott18. This long deposition and the interference with bone remodelling should be taken into account before the initiation of this therapy. As these drugs are eliminated (almost exclusively) by the kidney, renal function after kidney transplantation has also to be considered. In this patient, the bisphosphonates prescribed by the gynaecologist for osteoporosis treatment potentially could lower serum calcium concentration, but hypercalcaemia persisted. In face of persistent hypercalcaemia with elevated PTH, cinacalcet was introduced. Several small studies showed that cinacalcet was able to lower the serum calcium, with no significant change in the PTH values19-21.
BMD provides no information regarding bone quality, bone mineralization, bone architecture or turnover in RT population22. Nevertheless, KDIGO suggests to measure BMD in the first 3 months after RT when patients have GFR > 30 mL/min/1.73m2 (evidence grade 2D) and, when low, to consider treatment with vitamin D, calcitriol/alfacalcidiol or bisphosphonates (evidence grade 2C). They also refer that treatment choices may be influenced by the presence of CKD–MBD, as indicated by abnormal levels of calcium, phosphorus, PTH, alkaline phosphatases and 25(OH)vitamin D.
KDIGO guidelines also defend that it is reasonable to consider the need to perform a bone biopsy to guide treatment (Not Graded)23. The Spanish Nephrology Society guidelines launched 2 years ago go even further on that issue: they recommend a DEXA scan to kidney recipients with fracture risk and, if it reveals osteoporosis and PTH is > 60 pg/ml, to start bisphosphonates; they also recommend calcimimetics after RT if serum calcium > 10.5 mg/dl and iPTH > 100 pg/ml24. Of note, the KDOQI US commentary on KDIGO guidelines already stated that bisphosphonates should not be used if there are abnormalities in calcium, phosphate, vitamin D, or PTH levels25.
Hypercalcaemia
Hypercalcaemia after kidney transplantation is common and often transient in the first 6 months, but cannot fade away in some patients. Tubulointerstitial calcifications in the kidney graft have been demonstrated in RT patients with high serum PTH and calcium levels in a well-known study, which correlated with an inferior graft outcome one year after transplantation, and hyperparathyroidism and hypercalcaemia were identified as the key factors26.
Of note, phosphate supplementation was more prevalent in patients with tubulointerstitial calcifications; moreover, in a subsequent study with 3.5 times more patients, high calcium levels were associated with a reduced risk for allograft loss27.
Usually hypercalcaemia is due to hyperparathyroidism that persists from the previous pre-RT period, and results from the direct effect of PTH in causing calcium efflux from the bone now added to the enhanced renal tubular absorption of calcium by PTH action in the functioning kidney graft, and to the effects of higher levels of calcitriol on the gastrointestinal absorption of calcium by the restoring of 1 α-hydroxylase activity subsequent to functioning nephron mass.
Hyperparathyroidism
In up to 50% of transplant recipients, abnormal PTH secretion persists, sometimes causing hypercalcaemia that may require parathyroidectomy28. Cinacalcet has been shown to correct hypercalcaemia and hyperparathyroidism in several trials in kidney transplant recipients19,20,29-36, however, few of those studies had a control group and none had bone biopsies. One of the concerns with calcimimetics is nephrocalcinosis and graft dysfunction (3 case reports in the literature)37-39.
In a recent study by Courbebaisse et al., 34 patients received cinacalcet between 3 to 12 months after RT; hypercalciuria was more than twice as high at month 12 as in patients who did not receive cinacalcet treatment, but that was not associated with an increase in calcium deposits on renal biopsies or an alteration of measured GFR30.
Biochemical parameters of bone metabolism, although somewhat useful in monitoring bone health in the non-renal transplant patient, do not predict bone activity or mineralization in the renal transplant recipient, suggesting a skeletal resistance to PTH and eventually also to active vitamin D340-42. Nevertheless, some experts suggest that management of post-transplantation bone disease should initially focus on correcting metabolic disorders, using the same principles applied to manage CKD–MBD prior to RT2, 23,24, and consider bone biopsy as an important, yet eventual, additional tool. The remarkable clinical evolution in this case report, causing high morbidity and affecting the quality of life of this transplant recipient, which greatly improved after changing therapeutic strategy, acknowledges that an improved BMD may not reflect an improvement on bone health. These results also underline that adynamic bone histology may coexist with high iPTH serum levels in the transplanted patients (as already described in the dialysis population43,44).
Recognition of MBD in RT patients as a unique disorder is critical to provide appropriate therapy and avoid iatrogenesis. We believe that the new version of KDIGO guidelines will update current guidelines on this topic.
CONCLUSIONS
Especially after RT, biochemical hyperparathyroidism is a complex entity that can be associated with high or low bone turnover. Treatment of persistent hypercalcaemia according to some prevailing recommendations can lead to, or exacerbate, adynamic bone disease. Presently, it is not known if and at what calcium level treatment should be initiated.
In the absence of any randomized controlled trial for the treatment of renal osteodystrophy after RT, it seems wise to begin the therapeutic approach by the correction of deficiency/insufficiency of vitamins, hormones and minerals as we did in the present situation, through the supplementation of cholecalciferol.
Our findings also suggest that before the use of a long acting drug (like bisphosphonates) or an off label drug (like cinacalcet) in a transplanted patient, a bone biopsy should be performed to evaluate bone characteristics: volume, mineralization, quality and architecture (with separation between cortical and trabecular bone).
The presented case draws attention to the fact that we must focus on the trigger event, instead of its manifestations.
References
1. http://www.kdigo.org [ Links ]
2. Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis 2013;61(2):310-325 [ Links ]
3. Weisinger JR, Carlini RG, Bellorin-Font E. Bone disease after renal transplantation. Clin J Am Soc Nephrol 2006;1(6):1300-1313 [ Links ]
4. Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab 2005;90(4):2456–2465 [ Links ]
5. Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millenniumanalysis of 630 bone biopsies in black and white patients. J Bone Miner Res 2011;26(6):1368-1376 [ Links ]
6. Govindarajan S, Khandelwal N, Sakhuja V, Jha V. Bone mineral density in patients with end-stage renal disease and its evolution after kidney transplantation. Indian J Nephrol 2011;21(2):85-89 [ Links ]
7. Brandenburg VM, Politt D, Ketteler M, et al. Early rapid loss followed by long-term consolidation characterizes the development of lumbar bone mineral density after kidney transplantation. Transplantation. 2004;77(10):1566-1571 [ Links ]
8. Pichette V, Bonnardeaux A, Prudhomme L, Gagné M, Cardinal J, Ouimet D. Long-term bone loss in kidney transplant recipients: a cross-sectional and longitudinal study. Am J Kidney Dis 1996;28(1):105-114 [ Links ]
9. Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 1991;325(8):544-550 [ Links ]
10. Jeffery JR, Leslie WD, Karpinski ME, Nickerson PW, Rush DN. Prevalence and treatment of decreased bone density in renal transplant recipients: A randomized prospective trial of calcitriol versus alendronate. Transplantation 2003;76(10):1498–1502 [ Links ]
11. Haas M, Leko-Mohr Z, Roschger P, et al. Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 2003;63(3):1130–1136 [ Links ]
12. Grotz W, Nagel C, Poeschel D, et al. Effect of ibandronate on bone losss and renal function after kidney transplantation. J Am Soc Nephrol 2001;12(7):1530-1537 [ Links ]
13. Fan SL, Almond MK, Ball E, Evans K, Cunningham J. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int 2000;57(2):684–690 [ Links ]
14. Torregrosa JV, Fuster D, Pedroso S, et al. Weekly risedronate in kidney transplant patients with oeteopenia. Transpl Int 2007;20(8):708-711 [ Links ]
15. Mitterbauer C, Schwartz C, Haas M, Oberbauer R. Effects of bisphosphonates on bone loss in the first year after renal transplantationa meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2006;21(8):2275-2281 [ Links ]
16. Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev 2007;18(3):CD005015 [ Links ]
17. Coco M, Glicklich D, Faugere MC, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 2003;14(10):2669-2676 [ Links ]
18. Ott SM. Therapy for patients with CKD and low bone mineral density. Nat Rev Nephrol 2013;9(11):681-692 [ Links ]
19. Srinivas TR, Schold JD, Womer KL, et al. Improvement in hypercalcemia with cinacalcet after kidney transplantation Clin J Am Soc Nephrol 2006;1(2):323– 326 [ Links ]
20. Serra AL, Schwarz AA, Wick FH, Marti HP, Wüthrich RP. Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrol Dial Transplant 2005;20(7):1315–1319 [ Links ]
21. Kruse AE, Eisenberger U, Frey FJ, Mohaupt MG. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrol Dial Transplant 2005;20(7):1311–1314 [ Links ]
22. Grotz WH, Mundinger FA, Gugel B, Exner V, Kirste G, Schollmeyer PJ. Bone fracture and osteodensitometry with dual energy x-ray absorptiometry in kidney transplant patients. Transplantation 1994;58(8):912-915 [ Links ]
23. Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(Suppl 3):S1-S155 [ Links ]
24. Torregrosa JV, Cannata Andia J, Bover J, et al. Recomendaciones de la Sociedad Española de Nefrología para el manejo de las alteraciones del metabolismo óseomineral en los pacientes con enfermedad renal crónica (S.E.N.-MM). Nefrologia 2011;31(Suppl 1):3-32 [ Links ]
25. Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD–Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 2010;55(5):773-799 [ Links ]
26. Gwinner W, Suppa S, Mengel M, et al. Early calcification of renal allografts detected by protocol biopsies: Causes and clinical implications. Am J Transplant 2005;5(8):1934–1941 [ Links ]
27. Schaeffner ES, Födinger M, Kramar R, Sunder-Plassmann G, Winkelmayer WC. Prognostic associations of serum calcium, phosphate and calcium phosphate concentration product with outcomes in kidney transplant recipients. Transpl Int 2007;20(3):247–255 [ Links ]
28. Cohen JB, Gordon CE, Balk EM, Francis JM. Cinacalcet for the treatment of hyperparathyroidism in kidney transplant recipients: a systematic review and meta-analysis. Transplantation 2012;94(10):1041-1048 [ Links ]
29. Pinho LR, Ribeiro Santos MJ, Pestana Vasconcelos M. Cinacalcet in the treatment of persistent hyperparathyroidism after kidney transplantation. Clin Nephrol 2011;75(3):263-268 [ Links ]
30. Courbebaisse M, Diet C, Timsit MO, et al. Effects of cinacalcet in renal transplant patients with hyperparathyroidism. Am J Nephrol 2012;35(4):341-348 [ Links ]
31. Bergua C, Torregrosa JV, Fuster D, Gutierrez-Dalmau A, Oppenheimer F, Campistol JM. Effect of cinacalcet on hypercalcemia and bone mineral density in renal transplanted patients with secondary hyperparathyroidism. Transplantation 2008;86(3):413-417 [ Links ]
32. El-Amm JM, Doshi MD, Singh A, et al. Preliminary experience with cinacalcet use in persistent secondary hyperparathyroidism after kidney transplantation. Transplantation 2007;83(5):546-549 [ Links ]
33. Serra AL, Savoca R, Huber AR, et al. Effective control of persistent hyperparathyroidism with cinacalcet in renal allograft recipients. Nephrol Dial Transplant 2007;22(2):577-583 [ Links ]
34. Szwarc I, Argilès A, Garrigue V, et al. Cinacalcet chloride is efficient and safe in renal transplant recipients with posttransplant hyperparathyroidism. Transplantation 2006;82(5):675-680 [ Links ]
35. Leca N, Laftavi M, Gundroo A, et al. Early and severe hyperparathyroidism associated with hypercalcemia after renal transplant treated with cinacalcet. Am J Transplant 2006;6(10):2391-2395 [ Links ]
36. Leonard N, Brown JH. Persistent and symptomatic post-transplant hyperparathyroidism: a dramatic response to cinacalcet. Nephrol Dial Transplant 2006;21(6):1736 [ Links ]
37. Peng LW, Logan JL, James SH, Scott KM, Lien YH. Cinacalcet-associated graft dysfunction and nephrocalcinosis in a kidney transplant recipient. Am J Med 2007;120(9):e7-e9 [ Links ]
38. Esposito L, Rostaing L, Gennero I, Mehrenberger M, Durand D, Kamar N. Hypercalciuria induced by a high dose of cinacalcet in a renal-transplant recipient. Clin Nephrol 2007;68(4):245-248 [ Links ]
39. Seikrit C, Mühlfeld A, Groene HJ, Floege J. Renal allograft failure in a hyperparathyroid patient following initiation of a calcimimetic. Nat Rev Nephrol 2011;7(4):237-241 [ Links ]
40. Carlini RG, Rojas E, Weisinger JR, et al. Bone disease in patients with long-term renal transplantation and normal renal function. Am J Kidney Dis 2000;36(1):160-166 [ Links ]
41. Montalban C, De Francisco AL, Mariñoso ML, et al. Bone disease in long-term adult kidney transplant patients with normal renal function. Kidney Int 2003;63(Suppl 85):S129–S132 [ Links ]
42. Borchhardt K, Sulzbacher I, Benesch T, Födinger M, Sunder-Plassmann G, Haas M. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant 2007;7(11):2515-2521 [ Links ]
43. Ferreira A, Frazão JM, Monier-Faugere MC, et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol 2008;19(2):405-412 [ Links ]
44. Barreto FC, Barreto DV, Moysés RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 2008;73(6):771-777 [ Links ]
Drª Rita Birne
Nephrology Department, Hospital de Santa Cruz
Av. Prof. Reinaldo dos Santos
2790-134 Carnaxide, Portugal
E-mail: r_birne@yahoo.com
Conflict of interest statement: None declared.
Received for publication: 09/11/2013
Accepted in revised form: 04/02/2014














