Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.3 Lisboa set. 2014
ORIGINAL ARTICLE
Fluid management in haemodialysis: Conventional versus Body Composition Monitoring (BCM) supported management of overhydrated patients
Prescrição do Peso Seco em Hemodiálise: Controlo da hiperhidratação comparando a avaliação clínica vs determinação da composição corporal por bioimpedância
Pedro Ponce1, Jenny Pham2, Olivera Gligoric-Fuerer3, Ursula Kreuzberg3
1 Nephrocare Portugal, National Medical Coordinator. Lisboa, Portugal
2 Fresenius Medical Care Deutschland GmbH, Research & Development / Clinical Research. Bad Homburg, Germany
3 Fresenius Medical Care Deutschland GmbH, International Medicine and Marketing, Medical Affairs & Medical Information. Bad Homburg, Germany
ABSTRACT
Introduction: Normohydration is an important target in dialysis patients. In this study, we compared the performance of a bioimpedance spectroscopy device versus conventional clinical judgment in assessing the hydration status of HD patients and determining their ideal weight. Materials and Methods: 189 HD patients on online haemodiafiltration participated in this prospective, controlled, multicentre study. Dialysis units were randomly divided into an open-access and a blinded BCM group. Hydration status, blood pressure, weight gain, morbidity and mortality were assessed. Results: At baseline, 92 patients of the BCM-open group and 79 patients of the BCM-blinded group were overhydrated by ~3.8L. After one year, the rate of patients with OH > 2.5L was reduced to 52.5% in the open, and to 65.9% in the blinded group. Hospitalization and survival rate was not significantly different between the two groups. Conclusion: Results suggest that BCM is a helpful tool in supporting the fluid management of HD patients.
Key-Words: BCM;blood pressure; dry weight; haemodialysis; overhydration.
RESUMO
Introdução: A obtenção de um estado de normo-hidratação é um dos alvos mais relevantes na prescrição de diálise. Comparamos neste estudo a performance de um equipamento de espectroscopia de bioimpedância (BCM – body composition module) versus a avaliação clínica convencional para a determinação e prescrição do Peso Seco em doentes em hemodiálise. Material e Métodos: 189 doentes tratados por HDF online participaram neste estudo prospectivo, aleatório, multicêntrico. As unidades de diálise participantes foram aleatoriamente divididas em um grupo de acesso aberto aos resultados do BCM e um grupo cego para estes resultados. Foram determinados mensalmente, ao longo de 1 ano, em todos os doentes, o estado de hidratação, pressão arterial, ganho de peso inter-dialítico, a morbilidade e a mortalidade.
Resultados: No inicio do estudo, 92 doentes no grupo aberto aos resultados do BCM e 79 doentes do grupo cego estavam hiperhidratados em média 3.8L acima do seu peso ideal. Após 1 ano, a percentagem de doentes com hidratação > 2.5L pré-diálise reduziu-se a 52.5% no grupo aberto e a 65.9% no grupo fechado. A morbilidade (hospitalizações) e mortalidade não variou significativamente entre os 2 grupos. Conclusão: O BCM revelou ser uma ferramenta útil como suporte à prescrição e obtenção do peso ideal em doentes em hemodiálise.
Palavras-chave: BCM; hemodiálise; hiperhidratação; peso seco; pressão arterial.
INTRODUCTION
Chronic volume overload in haemodialysis patients has been related to hypertension, heart failure, left ventricular hypertrophy, and other adverse cardiovascular outcomes, as shown in several studies1. A recent study by Chazot et al.2 demonstrated, after a follow-up of 6.5 years, that reaching a correct fluid target has more impact on survival than prolonged treatment time. Involved in this study were 55 patients in two dialysis units, who were submitted to one cross-sectional measurement of hydration status and were stratified on hyper-or normohydration. In another study, Chazot et al.3 discovered that early correction of blood pressure by fluid removal erased the reverse epidemiology of blood pressure and improved patient survival. A further cross-sectional analysis of fluid status in 269 patients, with a follow-up of 3.5 years, identified the hydration state as an independent predictor of mortality in chronic dialysis patients, secondary only to the presence of diabetes4.
Therefore, the achievement of a normal hydration state in patients is one of the most important goals of haemodialysis treatment. The concept of dry weight is part of the daily practice of prescribing nephrologists. Dry weight is defined as the lowest weight a patient can tolerate without developing hypotensive episodes. This assessed dry weight constitutes the base for the prescription of the ultrafiltration goal.
Despite its apparent usefulness, the definition of patients dry weight is rather an abstract and quite imprecise concept, as shown in previous studies5-9.
To determine the hydration status and prescribe dry weight, clinical parameters are used such as interdialytic weight gain, blood pressure, the presence of oedema, or historical tolerance to ultrafiltration10.
For improved prediction of a target endpoint for the normal hydration state, a reliable and in particular quantified method for the assessment of individual euvolaemia in kilograms is strongly needed.
The EBPG guidelines on haemodialysis instability, in guideline 1.1.2, recommends objective methods to assess fluid state in patients with frequent intradialytic hypotension, or when clinical examination is inconclusive (level III).
In this present study we used a bioimpedance spectroscopy method (Body composition monitor (BCM®), Fresenius Medical Care, Bad Homburg, Germany).
The BCM calculates body composition, including extracellular water (ECW) and intracellular water by measuring bodys resistance and reactance to electrical current. This non-invasive tool delivers within two minutes not only the fluid status of a patient but also body composition parameters, like lean and adipose tissue mass.
The bioimpedance spectroscopy device (BCM-Body composition module) was extensively validated both in vitro and clinically in healthy subjects and dialysis patients11,12.
The aim of this real-life study was to compare the performance and safety of a bioimpedance spectroscopy device versus conventional clinical judgment as used in our daily practice. Our goal was to find out if there was any advantage in using the BCM tool compared to conventional clinical judgment for preventing overhydration (OH) and avoiding intradialytic hypotensive episodes in a cohort of overhydrated patients.
Secondary objectives were the comparison of blood pressure control and its correlation with the hydration status, morbidity and mortality between groups.
PATIENTS AND METHODS
Study Design/Patients: This study was a prospective, multicentre trial. Overhydrated dialysis patients, treated with online haemodiafiltration (HDF) in 23 Portuguese dialysis units participated in this study and were evenly divided among aforementioned dialysis centres. Incident and prevalent HD patients were included if they were older than 18, with a relative predialytic overhydration (OH) (relative OH [%] = OH [l] / extracellular water [l]*100) at baseline of > 15% (on average > 2.5 litres) as assessed by the body composition monitor (BCM©). All patients were treated by three times weekly online HDF treatment of ≥ 4 hours per session.
Patients with an implanted electronic medical device or who were connected to an external electronic medical device were excluded. Further exclusion criteria were: Any kind of metal implants or metal prosthetic joints, e.g., implanted defibrillators, cardiac pacemakers.
On the other hand, dental implants and piercings were allowed. Patients with major amputations, pregnant women, and patients with symptomatic aortic valve stenosis were also excluded (Fig. 1).
The 23 participating dialysis units were randomly assigned to an open-access BCM group (n = 101 patients; 53.4%) or a blinded BCM group (n = 88 patients; 46.7%). The investigation was performed between 2010 and 2012. Each patient was under observation for one year, during which 12 monthly assessments were accomplished.
Only assessments with available treatment and BCM data were accepted for analyses. In order to match the assessments to the monthly visit scheme, assessments within ± 15 days from schedule were tolerated. For baseline, the range was extended up to -30/+15 days and for the 12-month-visit, an extended range of -15/+30 days was used. In case of multiple assessments within the tolerance frame, the one with the least difference to the foreseen visit was used.
All participating Portuguese centres are fully registered in EuClid® (European Clinical Database), a patient registry and data management system run by Fresenius Medical Care in its own European dialysis centres. All baseline patient data were taken from this database.
Informed consent from all patients was requested, and patients confidentiality was respected throughout.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Assessment of hydration status
According to BCM development and validation studies8,12, nomohydration is defined when absolute fluid overload is between the 10th and the 90th percentile for healthy individuals from a reference population, i.e., between -1.1 and +1.1L, while volumes below or above this range define under or overhydration, respectively. When extra-cellular water overhydration, as define above, is higher than 15%, that means that, on average, the fluid overload is above 2.5L, what defines severe overhydration, the inclusion criteria in our study.
In summary, we classify HD patients according to their hydration status in four categories: Patients were underhydrated with OH ≤ -1L, they were normally hydrated with -1L < OH ≤ 1L, mildly overhydrated with 1L < OH ≤ 2.5L and severely overhydrated if OH > 2.5L.
In both, the open and the blinded centres, the hydration status of patients was measured once monthly by the BCM at midweek dialysis treatment, prior to dialysis session. Data of pre-dialysis measurements, however, were only accessible to the treating physicians of the open access BCM centres, the patients fluid status as measured by BCM was not communicated to physicians or nurses in the blinded centres. In the latter, a research nurse was in charge of documenting the fluid status measurements of each patient, ensuring that physicians were blinded to the results. Physicians in the blinded group units used all conventional fluid management techniques according to traditional centre standards, in order to assess dry weight of their patients and to adjust ultrafiltration.
Assessment of other parameters
Monthly at midweek dialysis treatments, together with BCM hydration status, pre-and post-dialytic weights were assessed. Additionally, blood pressure was determined before and after dialysis treatment. All intradialytic hypotension events were registered if systolic blood pressure was reduced by > = 30mmHg during dialysis or if the systolic blood pressure dropped intradialytically below 90mmHg.
During the assessment period, hospital admissions and mortality were recorded. Thereby both, first hospital admission and mortality associated with cardio- and cerebro-vascular disease were counted as events.
Statistics/analysis
For all statistical analyses, only those patients who met the inclusion without any of the exclusion criteria were included.
For the primary analysis, group differences at study end were analysed via baseline-adjusted ANalysis of COVAriance (ANCOVA). For subgroup analyses, the ANCOVA was extended by the respective subgroup as additional factor.
Group-specific differences between baseline and study end were assessed via paired samples t-test.
Chi-squared tests and Wilcoxon tests were applied to investigate safety issues like hypotensive events.
For the primary analysis (absolute OH) missing values were imputed using the last observation carried forward (LOCF) principle.
The significance level was set to α = 0.05. Since the subgroup analyses had exploratory character only, no adjustment for multiplicity was regarded as necessary.
Descriptive statistics are given as mean ± standard deviation, if not stated otherwise.
All analyses were performed with the software package SAS V9.2.
RESULTS
Study Participants
Of 218 initially recruited potential participants, 189 patients met the inclusion criteria for this study.
Patients excluded were either not consistently overhydrated in the run-in period (n = 10) or their dialysis treatment time was less than 4h/session (n = 14).
Seventy-one patients prematurely terminated the study: 29 patients of the open-label group, and 42 of the blinded group. Of these patients, 35% did not have a valid assessment within the predefined time frame, and 28% (n = 20) died (Fig. 1).
Patients in both groups were of similar age (openlabel: 65.82 ± 14.56 years; blind: 66.70 ± 15.10 years), the majority were of male sex (open-label: 71.3%; blind: 81.8%). Patient data on body height and weight, as well as the body mass index (BMI) prior to study start were identical. Hypertension was the most frequent comorbidity (open-label: 72.3%; blind: 73.9%), followed by diabetes mellitus (open-label: 38.6%; blind: 39.8%).
Other comorbidities, like congestive heart failure, myocardial infarction or coronary artery disease, had prevalence of less than 25% in each group. The primary cause for renal disease was diabetes nephropathy (open-label: 30.7; blind: 35.2%; Table 1)
Hydration status
At baseline, all patients were overhydrated.
However, 92 patients (91.1%) of the open label and 79 patients (89.8%) of the blinded group were severely overhydrated. Patients of both groups started with a similar overhydration of ~3.8L at baseline and the overhydration status was significantly reduced in both groups by study end.
Patients in the open label group started with a mean overhydration status of 3.77 ± 1.23L (OH[L], corrected for weekday) and the blinded group started with a mean overhydration of 3.81 ± 1.38L (OH[L], corrected for weekday). At month 12, patients of the open access group showed a mean hydration level of 2.92 ± 1.47L and in the blinded group mean overhydration was at 3.36 ± 1.75L (Fig.2). This difference in overhydration between both groups reached borderline significance at month12 (estimate for blind-open 0.4184 L(95% CI: [-0.02 – 0.86], p = 0.0622) Accordingly, at end of study the absolute OH[L] was reduced in HD patients of the open label group to 52.5% (in the category severely overhydrated) and in those of the blinded control to 65.9% (in the same category).
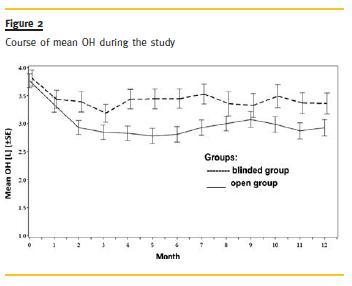
Relative overhydration (OH[%], as measured by BCM) improved over time: At baseline 20.65 ± 4.68% of HD patients in open access group and 20.73 ± 5.73% patients of the blinded group were overhydrated. At the end of study, the open group showed a mean overhydration value 15.40 ± 6.36% and the control 16.26 ± 8.48%.
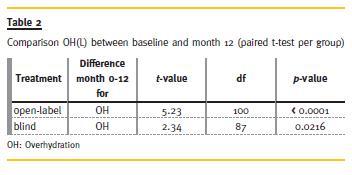
The reduction of OH after 12 months compared to baseline was significant in both groups (p = <0.0001 in the open cohort, and p = 0.0216 for the blinded cohort, paired t-test per group; Table 2).
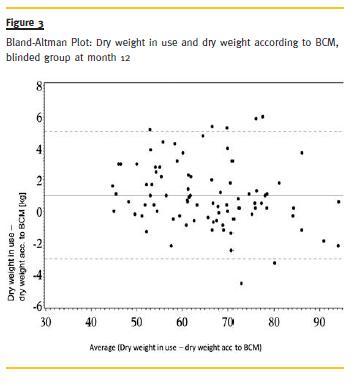
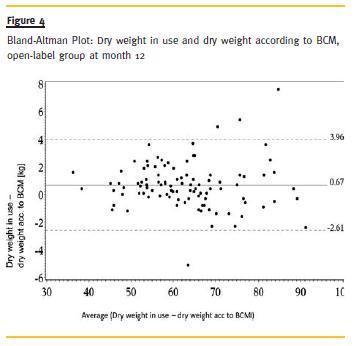
Dry weight in use and dry weight according to BCM were highly correlated throughout the study (r =.99). Bland-Altman-Plots at month 12 revealed that dry weight in use is generally less overestimated in the open group than in the blinded group (0.67 vs. 1.00 kg). In the open group a higher accordance in terms of variation could be achieved (Fig. 3 and Fig. 4).
Blood pressure (BP)
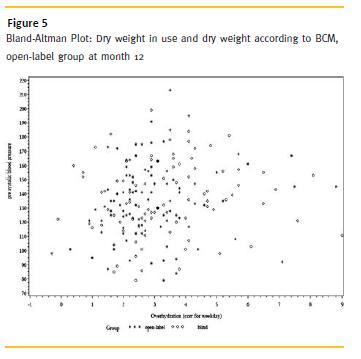
Both groups started with a similar pre- and postdialytic systolic and diastolic BP, in both cohorts the values declined until month 12. In the open cohort the pre-dialytic systolic BP was reduced from 144.8 ± 24.1 mmHg to 134.6 ± 27.3 mmHg (in the blind group: from 145.9 ± 26.8 mmHg to 136.5 ± 24.7 mmHg) and pre-dialytic diastolic BP from 68.3 ± 14.4 mmHg to 65.4 ± 15.8 mmHg (in the blind group: 69.7 ± 16.7 mmHg to 64.5 ± 16.2 mmHg). The postdialytic systolic BP in the open-access group changed from 145.0 ± 25.4 mmHg to 132.8 ± 28.6 mmHg and diastolic blood pressure from 68.8 ± 14.1 mmHg to 63.4 ± 15.0 mmHg, respectively. In the blind group, comparable changes in BP were observed: Systolic BP declined from 142.5 ± 29.4 mmHg to 129.3 ± 24.0 mmHg, and diastolic from 66.1 ± 14.2 mmHg to 61.4 ± 12.9 mmHg (Fig. 5).
Hypotensive events
In this study the difference in frequency of hypotensive events that occurred in both groups was not significant – so was the number of patients suffering from at least one event. At baseline, 39 hypotensive events were observed in 17 patients in the open-label group, compared to 28 events in 12 patients in the blinded group. At the end of study, at month 12, 48 hypotensive events were observed in 20 patients of the open-label group, compared to 41 events in 15 patients in the blinded group.
Death/hospitalizations:
In the open-label group, 40 (39.6%) off the 101 patients were hospitalized at least once, and in the blinded group, 28 (31.8%) off 88 patients.
During the study period, 20 patients died, thereof nine patients died in hospital. Forty per cent (eight) of the death cases occurred in the blind group, of which in four cases the death reasons were not further specified; three patients died of acute myocardial infarction, and one patient died of sepsis. In the remaining 12 death cases that occurred in the open-label group, the patients died of acute myocardial infarction, mesenteric ischaemia, unspecific cardiac arrest, cerebral infarction, chronic respiratory failure, prostate carcinoma and pulmonary embolism.
Two patients died of unspecified septicaemia and in the remaining three patients the death cause was unspecified.
We further investigated whether there is a difference between patients with a dry weight in accordance with the dry weight suggested by BCM (±500g) and outside this range, regarding time to first hospitalization or time to death. The survival probability was slightly higher in the group outside the range than in the group within±500g. But this difference was not significant (Fig. 6).

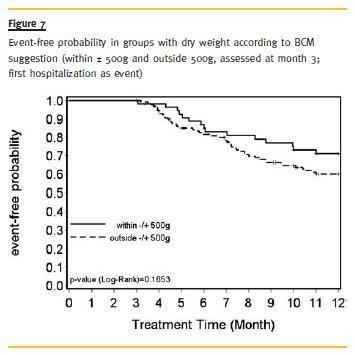
Regarding hospitalization, patients within ±500g had a higher chance of hospitalization-free time, although this difference was not significant either (Fig. 7).
DISCUSSION
Fluid management is crucial to the treatment ofend-stage chronic renal failure, to improve cardiovascular tolerance to dialysis treatment, quality of life and survival. In fact, mortality increases beyond a sustained pre-dialysis fluid overload level of around 2.5 litres2,4.
The results of our study indicate that the hydration status of HD patients can be improved over time by tight monitoring.
Our results indicate that bioimpedance spectroscopy measurements with the BCM module was advantageous in the fluid management of our ESRD population, because after 12 months patients of the open-label group had a clear trend for better fluid control. The severe overhydration in 92 patients at baseline, was reduced in almost half of the patients(n = 39) within this category. Although similar results were observed in the blinded group, demonstrating good clinical performance, at least during a study, the BCM seemed to be a helpful diagnostic tool that reasonably complements existing clinical methods in the management of overhydrated patients.
Petr Machek et al.13 in a cohort of overhydrated patients, with monthly BCM monitoring, managed to reduce patients fluid overload in 2L, resulting in a reduction of 25mmHg in the average systolic blood pressure and a 35% reduction in antihypertensive medication.
The correlation of fluid status and blood pressure has also been assessed in our study. The blood pressure in both groups was reduced as dry weight was decreased, even though not significantly. The monthly correlation between overhydration and predialytic systolic blood pressure, as well as pre-dialytic diastolic blood pressure varied without any specific pattern (r = 0.10-0.28 vs. r = 0.05-0.29, respectively), overall a marginal correlation and a wide scatter between blood pressure and overhydration.
These findings indicate that fluid overload is not necessarily associated with hypertension in the HD population, but that it is one more factor contributing to hypertension amongst others. In our series, patients in the blinded group with normal blood pressure were significantly more overhydrated at month 12, compared to the open group, an observation that supports the weakness of this correlation.
In another recent study of 72 stable ESRF patients in the UK, the authors also failed to show any correlation between multifrequency bioimpedance measurements and blood pressure, but there was a correlation with haematocrit, plasma albumin, and extracellular fluid volum14.
Onofriescu and co-workers in a recent pilot RCT also demonstrated a better control of fluid overload in a bioimpedance group compared to a control group with dry weight prescribed based on clinical grounds. Blood pressure did not change significantly in either group, but with a follow-up of 2.5 years they were able to show a reduction in mortality, with an HR for death of 0.11 in the bioimpedance group vs. the control15.
When looking at the whole patient population in our study, lumping together both groups, stratifying between patients attaining a dry weight within ± 500g from normohydrated weight and those persistently overhydrated, the time to first hospitalization was shorter in the persistently overhydrated group, though not significantly.
In our short follow-up of 1 year, we could not replicate a mortality benefit in our bioimpedance group, but again there was also an improvement in the fluid management of the control group.
As with our trial, previous studies suffer from important limitations. Beginning with the fact that we have no real gold standard to measure fluid overload to and use as a comparator16, others had no control group, or between groups randomization2,17, most had a design with a cross-sectional assessment of hydration status only at the beginning of the study4, or intensive fluid status optimization17 not feasible in real life long-term follow-up of dialysis patients.
CONCLUSION
Our results confirm a marginal better performance of fluid management when using BCM for assistance to prescribe dry weight. On the other hand, we have to consider that although the BCM recommendation is accurate for the ideal extracellular volume, clinically we may not always be able to reach that fluid status. Cardiovascular impairment and subsequent morbidity caused by end-organs hypoperfusion may occur if we try to decrease volume status as low as recommended, even if rightly so.
References
1. Wizemann V, Schilling M. Dilemma of assessing volume state. The use and the limitations of a clinical score. Nephrol Dial Transplant 1995;10(11):2114-2117. [ Links ]
2. Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant 2012;27(6):2404-2410 [ Links ]
3. Chazot C, Vo-Van C, Deleaval P, et al. Predialysis systolic blood pressure evolution in incident hemodialysis patients: Effects of the dry weight method and prognostic value. Blood Purif 2012;33(4):275-283 [ Links ]
4. Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009;24(5):1574-1579 [ Links ]
5. Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K. Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: A cross sectional-study. Nephrol Dial Transplant 2010;25(2):545-551 [ Links ]
6. Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: An overview. J Am Soc Nephrol 1999;10(2):392-403 [ Links ]
7. Levin NW, Zhu F, Keen M. Interdialytic weight gain and dry weight. Blood Purif 2001;19(2):217-221 [ Links ]
8. Kraemer M, Rod e C, Wizemann V. Detection limit of methods to assess fluid status changes in dialysis patients. Kidney Int 2006;69(9):1609-1620 [ Links ]
9. Hur E, Usta M, Toz H, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: A randomized controlled trial. Am J Kidney Dis 2013;61(6):957-965 [ Links ]
10. Levin NW, Zhu F, Keen M. Interdialytic weight gain and dry weight. Blood Purif 2001;19(2):217-221 [ Links ]
11. Moissl U, Bosaeus I, Lemmey A, et al. Validation of a 3C model for determination of body fat mass. J Am Soc Nephrol 2007;18:257A [ Links ]
12. Wabel P, Rode C, Moissl U, Chamney PW, Wizemann V. Accuracy of bioimpedance spectroscopy to detect fluid status changes in hemodialysis patients. Nephrol Dial Transplant 2007;22(Suppl 6):vi129 [ Links ]
13. Machek P, Jirka T, Moissl U, Chamney P, Wabel P. Guided optimization of fluid status in haemodialysis patients. Nephrol Dial Transplant 2010;25(2):538-544 [ Links ]
14. Booth J, Pinney J, Davenport A. Do changes in relative blood volume monitoring correlate to hemodialysis-associated hypotension? Nephron Clin Pract 2011;117(3):c179-c183 [ Links ]
15. Onofriescu M, Hogas S, Voroneanu L, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: A pilot randomized controlled trial. Am J Kidney Dis 2014;64(1):111-118 [ Links ]
16. Raimann J, Zhu F, Wang J, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int 2014;85(4):898-908 [ Links ]
17. Moissl U, Arias-Guillén M, Wabel P, et al. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol 2013;8(9):1575-1582 [ Links ]
Dr. Pedro Ponce
Nephrocare Portugal
Rua Professor Salazar de Sousa, Lote 12
1750-233 Lisboa
Portugal
E-mail: pedro.ponce@fmc-ag.com
Acknowledgements
The authors thank all the medical directors and the nursing staff of the participating dialysis units. Directors and units are namely: Vasco Miranda of Dinefro, João Paulo Oliveira of S.M. da Feira, Ilídio Rodrigues of Barreiro, Pedro Maia of Coimbra, Aníbal Ferreira of Vila Franca de Xira, Célia Gil of Dialverca, Leonídeo Dias of Ponte da Barca, Montalban of Covilhã, Pedro Ponce of Lumiar, Manuela Silva of V. N. Gaia, Joaquim Pinheiro of Fafe, José Vinhas of Setúbal, Carlos Oliveira of Almada, João Silva of SAMS, Pedro Neves of Faro, Jorge Pratas of Abrantes, Castro Henriques of Braga, João Cruz of Amadora, Rui Alves of Viseu, Galvão of Torres Vedras, Sequeira Andrade of Entroncamento, Viriato of Tavira, Teixeira de Sousa of Montijo, Fernando Neves of Santarém.
Conflict of interest statement: UK, JP, and OGF are employees of Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany, and PP is an employee of NephroCare Portugal, Lisboa, Portugal. All the dialysis units involved belong to the NephroCare – Portugal Network The authors declare that they have no other relevant financial interest.
Received for publication: 09/06/2014 Accepted in revised form: 29/08/2014














