Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.4 Lisboa dez. 2014
ORIGINAL ARTICLE
A prospective assessment of renal transplantation versus haemodialysis: which therapeutic modality is good value for society?
Uma avaliação prospectiva do transplante renal versus hemodiálise: na perspetiva social, qual a melhor modalidade terapêutica?
Margarida Domingos1, Miguel Gouveia2, João Pereira3, Fernando Nolasco1,4
1 Department of Nephrology, Hospital Curry Cabral, Lisbon, Portugal
2 Católica Lisbon School of Business and Economics, Portuguese Catholic University, Lisbon, Portugal
3 National School of Public Health, Nova University of Lisbon, Lisbon, Portugal
4 Faculty of Medical Sciences, Nova University of Lisbon, Lisbon, Portugal
ABSTRACT
Background: Economic evaluations help health authorities facing budget constraints. This study compares the health-related quality of life (HRQOL) and costs in patient subgroups on haemodialysis (HD) and renal transplantation (KT). Methods: In a prospective study with follow-up of 1-3 years, we performed a costutility analysis of KT vs. HD, adopting a lifetime horizon. A societal perspective was taken. Costs for organ procurement, KT eligibility, transplant surgery and follow-up of living donors were included. Key clinical events were recorded. HRQOL was assessed using the EuroQol instrument. Results: The HRQOL remained stable on HD patients. After KT, mean utility score improved at 3 months while mean EQ-VAS scores showed a sustained improvement. Mean annual cost for HD was 32,567.57. Mean annual costs for KT in the year-1 and in subsequent years were, 60,210.09 and 12,956.77 respectively. Cost for initial hospitalization averaged 18,740.74. HLA-mismatches increased costs by 75% for initial hospitalization (p < 0.001) and 41% in the year-1 (p < 0.05), and duplicate the risk of readmission in the year-1 (p < 0.05). The incremental costutility ratio was 5,534.46/QALY, increasing 35% when costs for organ procurement were added. KT costs were 41,541.63 more but provided additional 7.51 QALY. Conclusions: The KT is cost-effective compared with HD. Public funding should reflect the value created by the intervention and adapt to the organ demand.
Key words: Economic evaluation; haemodialysis; public funding; QALY; quality-adjusted life years; renal transplantation.
RESUMO
Introdução: As análises económicas aplicadas à saúde são fontes úteis de informação à alocação de recursos escassos. Este estudo compara a qualidade de vida e os custos em subgrupos de doentes em hemodiálise e transplantados renais. Métodos: No âmbito dum estudo prospectivo com follow-up de 1-3 anos, realizou-se uma análise custo-utilidade do transplante renal vs hemodiálise, na perspectiva da sociedade. O horizonte temporal escolhido foi o ciclo de vida dos doentes. Incluíram-se os custos da colheita de órgãos, seleção dos candidatos a transplante, cirurgia do transplante e follow-up dos dadores vivos. Registaram-se os eventos clínicos. Utilizou-se o EuroQol-5D na avaliação da qualidade de vida. Resultados: Não se observou variação da QVRS nos doentes em hemodiálise. Observou-se melhoria do índice de utilidade ao 3º mês de transplante e os valores na escala EQ-VAS melhoraram em todos os tempos de observação. O custo médio por doente em hemodiálise foi 32.567,57. O custo médio no 1º ano de transplante foi de 60.210,09 e nos anos seguintes 12.956,77. O custo médio do internamento inicial foi de 18.740.74. Cada incompatibilidade-HLA aumentou em 75% o custo do internamento inicial (p < 0.001) e em 41% o custo no 1º ano (p < 0.05), e duplicou o risco de internamento no 1º ano (p < 0.05). O rácio custo-utilidade incremental foi 5.534,46/QALY; a inclusão dos custos da colheita de órgãos para transplante agravou esse rácio em 35%. O transplante gera um acréscimo de 41.541,63 e um ganho adicional de 7,51 QALY. Conclusões: O transplante renal é custo-efetivo comparado com a hemodiálise; consequentemente, o financiamento deve refletir o valor criado pela intervenção e adequar-se à sua procura.
Palavras-Chave: Análise económica; anos de vida ajustados pela qualidade; financiamento público; hemodiálise; QALY; transplante renal.
INTRODUCTION
End-stage renal disease (ESRD) is a global public health problem. The societal impact of chronic kidney disease puts pressure on healthcare systems1. Portugal, with a population of 10 million inhabitants, is one of the European countries with the highest prevalence of ESRD (1,000-1,160 per million inhabitants)2.
An increase in ESRD is expected due to the ageing of the population, with the current proportion of 16% of the population aged over 65 projected to double in 20503, a prevalence of diabetic nephropathy of 7%, with 30% of these patients suffering from newly diagnosed ESRD4, and a high prevalence of hypertension (42%)5. Renal transplantation (KT) improves patient survival and health-related quality of life (HRQOL) and has a favourable cost-effectiveness ratio6. However, transplant eligibility and the availability of organs for transplantation constrain this treatment option. The impact of the newer immunosuppressive (IS) agents on cost-effectiveness and HRQOL is still unclear7. Few studies have used preference instruments to assess HRQOL in ESRD patients8. The absence of information about adverse events and hospitalizations is another major limitation of existing economic evaluations9. Transportation costs and lost productivity have rarely been included in the analysis10,11.
During 2012, 17,533 patients in Portugal received renal replacement therapy. About 60% were on publicly funded haemodialysis (HD) in private for-profit dialysis centres, 36% had a kidney transplant and 4% were on peritoneal dialysis12. This study aimed to evaluate HRQOL and costs of KT compared to HDin private dialysis-centres.
SUBJECTS AND METHODS
This prospective observational study was conducted in a single-centre for KT in Portugal, from 2008 to 2010. The study population included patients aged © 18 years, on chronic HD for at least three months, who were wait-listed for KT, and who had agreed to respond to the EuroQol-5D (EQ-5D) instrument, a generic preference-based questionnaire. The EQ-5D includes a classification system (EQ-5Dprofile) and a visual analogue scale (EQ-VAS). The EQ-5D profile includes five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression.
Responses record three levels of severity: no problems, some problems, and extreme problems, resulting in 243 possible sets of values. These unique health states are converted to a single summary index by applying scores from a standard set of preference weights, which were not available for the Portuguese population. As an alternative, utility values elicited from respondents in the United Kingdom were applied. The EQ-VAS records the respondents rating of his/her overall health status on a graduated, vertical visual analogue scale ranging from 0 to 100.
The HRQOL was assessed in HD patients (baseline) and two years after in those remaining on the transplant list. Follow-up interviews were scheduled at 3, 6, 12, 24 and 36 months after KT, including patients who had lost their graft. The questionnaire was self-administered.
At enrolment, demographic and socio-economic data were collected on: gender, age, marital status, household income, employment and education. Each transplant recipient acted as his/her own control on HD. Medical records were searched for the following information: primary cause of ESRD, time on HD, co-morbidities, clinical outcomes, medication (including IS regimens), sensitization to HLA-antigens (PRA), number of mismatches, extended criteria donor (ECD) kidneys, graft source (living/deceased), high immunological risk (< 2 donor-recipient compatibilities and PRA_25%). The ECD was defined as a deceased donor with a least one criterion: aged > 55 years, stroke as cause of death, history of hypertension or diabetes mellitus (DM) for at least 10 years, or cold ischemic time >24 hours)13. New-onset of diabetes Mellitus (NODAT)14, incidence of arterial hypertension, dyslipidemia, depression and neoplastic disease were included as adverse effects of IS.
The impact of KT on HRQOL was evaluated by comparing EQ-5D change scores from 2-years after KT to baseline. We investigated the associations between HRQOL and costs with patient characteristics and clinical events.
As a societal viewpoint was taken, both direct and indirect costs were included. Direct costs refer to all the resources consumed in delivering care to the patient, and were categorized into medical and non-medical costs. Indirect costs include lost productivity associated with early retirement and morbidity.
Costs were reported up to 2011, expressed in Euro () and assigned to HD and KT as follows.
Haemodialysis programme
Haemodialysis was considered the current clinical practice. Resource consumption was reported to the year prior to KT and assumed to be constant, annually.
National Health Service (NHS) payments to the private dialysis centres are based on a composite rate, at a price of 470.09/patient/week. This comprehensive price covers all services of HD including staff remuneration, medication (antihypertensive, anaemia and bone management agents), diagnostic procedures related to renal disease and management of vascular access.
Costs of hospitalizations were obtained from diagnosis-related groups (DRG).
Transportation costs were individualized, based on the price per Km paid by NHS, the distance between home and the HD centre and the number of transportations.
Lost productivity was valued by gross wages according to the human capital approach. We multiplied the sum of the base wage, overtime pay and regular benefits by 1.2375, to account for the Social Security contributive rate and the result by 14 months (including the vacation and Christmas subsidies) to obtain the annual salary (18,646.20). Productivity per working day was calculated from annual wages divided by 230, representing the effective days of work per year (excluding weekends and holidays).
Transplantation
Costs of organ donation
The costs associated with cadaveric donation were assumed to be for the following: a per diem rate at the Intensive Care Unit, histocompatibility tests obtained from hospital records and organ harvesting derived from DRG corresponding to nephrectomy.
The evaluation of a living donor candidate included consultations and diagnostic procedures. Hospitalization costs for living donation were derived from DRG data for nephrectomies. Living donor follow-up costs were based on individualized data.
Transplant recipients
The surgical procedure was valued by the price of ambulatory kidney surgery extracted from the national DRG database.
We applied mixed costing methods for the initial and subsequent hospitalizations. For the Transplant Unit, the main setting of admissions, a microcosting approach was applied. We collected individualized data for medication and diagnostic procedures (the main components of costs). Estimates of the average daily wage of health professionals were based on national remuneration tables, and the remaining daily costs were extracted from hospital records and multiplied by the length of hospitalization.
As hospitalizations in departments other than the Transplant Unit were uncommon, costs were derived from the national DRG database.
As specific drugs for transplanted patients are fully paid by the NHS, the doses and cost information were extracted from the hospital pharmacy database.
Costs of diagnostic procedures, consultations and urgency episodes were obtained from the national database, including reimbursements paid by NHS to hospitals.
Transportation costs and productivity changes were described on the HD programme.
Cost-utility of dialysis vs. renal transplantation
Once costs and benefits had been determined, a simple ratio of the mean incremental costs by the mean incremental utility scores provided the cost utility for HD relative to RT. In our economic model, data beyond the observation period was extrapolated.
Based on an ERA-EDTA cohort15 the projected life expectancy on dialysis and KT was assumed to be 6.7 and 16.4 years, respectively. An utility of zero was assigned to patients who died. We applied a three and five per cent per year discount rate to encompass the range typically employed in economic evaluations.
Sensitivity analysis
To explore the impact of uncertainty on our findings, a series of one-way sensitivity analysis were undertaken. Four factors were chosen: a 3% and 5% discount rate applied only to the costs (presenting QALYs in the undiscounted form), exclusion of productivity changes, equal life expectancy for HD and KT and inclusion of organ procurement costs.
Statistical Analysis
Descriptive statistics used were the arithmetic mean and 95% confidence intervals for normally distributed samples, and median with interquartile ranges for skewed variables. We used the students t-test and one-way ANOVA to compare means between continuous variables for normally distributed samples or Mann-Whitney U-tests for skewed samples. The Kruskal-Wallis test was used to compare mean differences between skewed variables in more than two groups. We used the Friedman test to investigate the presence of global changes in HRQOL during the study period, and the Wilcoxon test was used to identify differences of EQ-5D utility and EQ-VAS scores between paired time observations using the Bonferroni correction. We used multiple regression models to identify significant predictors of HRQOL and costs. Statistical significance was assumed for p values < 0.05. We used the software Statistical Package for Social Sciences, version 13.0 for Windows.
RESULTS
Population
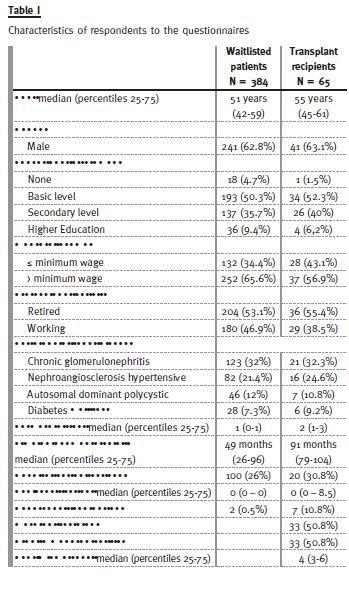
Of 384 wait-listed patients enrolled, sixty-five underwent KT. Their characteristics are summarized in Table I. Baseline IS for transplanted patients consisted of steroids, tacrolimus and mycophenolate mofetil. Induction IS included Basiliximab to patients at low immunologic risk (49%) and polyclonal antibody for those with high immunologic risk (51%).
HRQOL on HD
At baseline, mean EQ-5D utility was rated at 0.75; women reported a lower value (p < 0.001). Mean EQ-VAS score was 63.54; diabetics reported a lower value (p < 0.05). A second evaluation of HRQOL was requested from 165 patients remaining on the transplant list. The response rate was 50%. Gender, age, education level and wage did not differ between respondents and non-respondents. There was no change in HRQOL between the two interviews. Multiple regression analyses failed to show relationships between HRQOL measures and baseline variables.
Changes in EQ-5D utility and EQ-VAS scores after KT
Transplanted patients had a significant change of EQ-5D utility (p < 0.01) and EQ-VAS scores (p < 0.001) over time; in comparison with HD, median utilities improved at 3-months after KT (p < 0.01) with no significant change thereafter, and median EQ-VAS showed a sustained improvement (p < 0.001).
Median EQ-5D utility was higher on successful transplantation compared to renal allograft loss: 0.85 vs. 0.42 in the first year (p < 0.05), and 0.92 vs. 0.19 in subsequent years (p < 0.01), respectively.
Compared to the baseline, the evaluation of HRQOL 2-year after KT showed no significant change in EQ-5D utility, although there was a change on EQ-VAS (p < 0.001). The EQ-VAS change score averaged 13.94 (95% CI 8.47 to 18.45), reflecting an improvement of HRQOL.
Using a linear least squares regression, graft loss (β = – 0.719; p < 0.01), incidence of depression (β = – 0.181 p < 0.05), and NODAT (β = 0.227; p < 0.05) explained 22% (adjusted R2 = 0.223) of the variability of utility change scores. Hypersensitized patients (β = 17.175; p < 0.05), autosomal dominant polycystic disease (β =18.576; p < 0.05) and IS switch (β = 17.348 p < 0.05) explained 28% (adjusted R2 = 0.284) of the variability of EQ-VAS change scores.
Responses to the EQ-5D dimensions showed that patients reported more problems with pain/discomfort and anxiety/depression on HD and KT. Pain/discomfort improved after KT.
Costs
The average annual cost of HD was 32,567.57/patient. Eighty per cent of this value was allocated to the comprehensive price (24,444.68), 6% both to transportation (1,863.16) and lost productivity (1,817.36), 4% to medication (1,301.55), and the remainder to diagnostic procedures and hospital admissions.
Costs of cadaveric and living renal donation were 4,796.17 and 6,051.30, respectively. The mean cost of follow-up of living donor in the first year was 823.29, reduced by half thereafter. The eligibility costs averaged 2,845.25 in the year of acceptance onto the transplant list and 1,184.33 in subsequent years.
Total costs on the first, second and third years of KT averaged 60,210.09, 12,956.77 and 11,778.65, respectively. Medication costs decreased in absolute amounts over time, accounting for 24% of the costs during the first year and 50-52% thereafter. Diagnostic procedures and consultations represented a constant proportion of annual costs (8-11%).
Hypersensitized patients (87,918.43 vs. 56,865.98; p < 0.01), high immunological risk (72,209.20 vs. 47,836.01; p < 0.001) and graft failure (84,030.92 vs. 59,132.53; p < 0.05) had higher costs at 1-year.
The mean primary hospitalization cost was 18,740.74 (median 15,348.86), representing 31% of 1-year costs. Other major components of costs were medication (10,745.88), other hospitalizations (5,494.89), diagnostic procedures (3,792.81) and consultations (3,691.70).
Sixty-two rehospitalizations occurred during the first year, 16 during the second year and three during the third year. The main causes were infections and graft dysfunction. Age, DM, HLA-mismatches and cardiac events during the initial hospitalization were significant predictors of 1-year readmission (p< 0.001) (Table II). Age decreases the risk of readmission by 9% per year (p < 0.05) while female gender and diabetics had nine and 24 times, respectively, the risk of being hospitalized (p < 0.05), HLA-mismatches duplicates (p < 0.05) and cardiac events carried six times the risk of readmission (p < 0.05).
Lost productivity caused by early retirement represented 6% of annual costs on HD, amounting to 1,817.36/patient. After KT, morbidity costs represented 2% of annual costs in the first year.
Changes in HRQOL and costs over time and its significant predictors are summarized in Table III.
Cost-utility of dialysis
Waiting on dialysis resulted in 0.76 utilities and this figure was assumed to be constant thereafter. As costs and utilities in the second and third years of KT did not differ significantly, our model considers that KT leads to 0.74 utilities during the first year and 0.77 thereafter. The incremental cost utility ratio (ICER) was 5,534.46/QALY (Table IV). Applying three and five per cent discount rate to costs and QALY, ICER was 3,764.76/QALY and 2,004.75 /QALY, respectively.
Sensitivity analysis
Applying a three and five per cent discount rate only to costs, ICER was 2,869.78/QALY and 1,263.98/QALY, respectively. When indirect costs were excluded, ICER was 5,436.41/QALY. KT was found to be dominant over HD when assuming equal survival as it yielded both lower costs (259,744.35 vs. 534,108.15) and higher benefits (12.59 QALY vs. 12.46 QALY). The inclusion of costs for organ procurement increased ICER by 35%.
DISCUSSION
This study shows that KT is cost-effective compared to HD, confirming previous studies. To our knowledge, this is the first cost-utility analysis of KT vs. HD performed in Portugal. Our study was based on prospectively collected data and included the assessment of direct utilities and both direct and indirect costs, namely: costs related to selection of candidates for KT, living and cadaveric donation, transportation, hospital admissions and productivity changes. We were able to determine the real burden of KT and HD on the healthcare system and society, and we identified subgroups of patients with differing risks after KT, which may help to improve the effectiveness of healthcare delivery.
Our research showed differences from that yielded by a previous cost analysis of ESRD treatment in Portugal16. Our methodology considered the additional issues: inclusion of both costs and health outcomes from a prospective observational study; collection of detailed cost data and measurement of patients preference-adjusted health status; extrapolation of costs and utilities beyond the study period over patient lifetime. Moreover, we calculate the ICER of KT vs. HD, allowing comparison across different health interventions in order to prioritize them.
In our study, ESRD patients rated their HRQOL higher compared to various international studies8,17.
Dialysis patients in Portugal may have better accessibility to healthcare, and nephrologists refer them to whatever specialized care they need, explaining the higher level of satisfaction with the provision of healthcare compared to the general population.
As earlier studies have demonstrated18, lower utility scores on HD were significantly associated with female gender and lower levels of schooling.
The HRQOL remained stable on HD patients. The perceived health by patients during HD is directly related to their expectations after KT19. The positive change of HRQOL observed at 3 months, in transplant recipients, reflects the sense of freedom from HD and the surge in well-being of patients shortly after KT. No other change of utilities occurred despite the occurrence of a sustained increase in EQ-VAS scores.
After KT, 22% of patients were prescribed antidepressants, and thus apparently did not experience the sustained improvement of HRQOL observed in a previous study20. On HD, due to the limitations imposed by illness and time to receive care, patients and their families accept a lower income generated by early retirement. After a successful transplant, a new reality emerges: there is plenty of time, diminished financial resources, and employment is only a remote possibility. No retired transplant recipient returned to work, corroborating the maintenance of professional activity on HD as the main predictor of employment after KT21. Working solutions are required, namely, a review of scheduled working time to reverse the high societal impact of lost productivity on ESRD. Taking equity considerations into account, a general wage rate was used instead of the actual wages of ESRD patients.
Patients with graft failure reported lower utilities.
The lowest utility values from patients who had lost their graft can generate a health state, rated by the general population as worse than death.
The costs during the first year (60,210.09) were higher compared to previous studies [6,22-24]. The inclusion of patients who had lost their graft, not stated in those studies, may explain this discrepancy.
We found higher costs in this specific subgroup that averaged 36,249.51 on initial hospitalization, 84,030.92 during the first year and 37,674.80 in subsequent years.
High-immunological risk patients presented higher costs at initial hospitalization and year-1. The chosen IS protocol, serial monitoring of donor-antibody and antibody rejection treatment could explain the difference but the favourable outcomes supported the use of the newer IS agents.
We found that HLA-mismatches increase the costs of initial hospitalization by 75% and 41% during the first year of KT, and carried a two-fold risk of readmission during the first year. A previous study noticed the relevance of HLA matching25.
Underfinancing of hospital services and organ procurement organizations impacts negatively on transplantation26. The specific DRG rate for KT is 9,296.58 , not covering the expenses incurred during the primary hospitalization. A revision of this price is a priority as the actual reimbursement model acts as a disincentive to expand KT programmes.
Using a predictive model to assess the risk of hospital readmissions may help to reduce future inpatient expenditures. In our study, the odds-ratio for readmission was 24 for diabetics and six for cardiac patients, suggesting the need of a programme targeted at patients with DM and cardiovascular disease. Preexisting cardiovascular disease proved to have a negative impact on HRQOL during the first two years of KT. It also adversely affected graft function in year-1, increased the occurrence of cardiac events on initial hospitalization and readmissions during the first year.
Although not an absolute contraindication for KT, these findings raise questions about the best treatment options for this specific subgroup.
The negative impact of ECD on morbidity (lower graft survival, higher costs and more rehospitalizations) was limited to the first year, supporting the old-for-old allocation on KT as, according to a previous study27, older people benefit from a lower waiting time to KT.
The incremental analysis showed that KT costs 41,541.63 more than HD but resulted in 7.51 additional QALY, representing seven years and five months of perfect health. The incremental cost-utility ratio was 5,534.46/QALY. The sensitivity analysis demonstrates the robustness of our conclusions. The assumption of equal life expectancy for HD and KT was the parameter with the greatest impact in our results.
This is a single centre study. Several factors support the generalizability of our findings to the national context: i) In Portugal, the provision of dialysis care is delivered by an organized referral network28. i) To meet parameters of clinical effectiveness, there are guidelines within the units of dialysis set down, enforced and currently audited by the health authorities, which means that there are few variations in clinical practice; ii) Listing into two transplant units minimized potential bias in the eligibility criteria to KT; iii) Although recruited from a single hospital, the studied population had different geographic and socioeconomic backgrounds.
This work has several strengths: a) As a longitudinal study, it allowed us to establish links between predictors and potential risk factors for HRQOL and costs; b) An intention-to-treat analysis was used, including patients with failed grafts and those who had died; c) EQ-5D utilities based on actual measurement can be incorporated into future economic evaluations; d) A micro-costing approach was used to estimate costs; e) Handling uncertainty with a series of one-way sensitivity analyses proved the robustness of the model outcomes which minimized the study limitations of having small sample size and being a single-centre analysis.
Using EQ-5D value set for United Kingdom can introduce a potential bias. EuroQol Group recommended its use because preferences weights for Portuguese population were not available.
We conclude that KT is cost-effective compared to HD, cost saving at 2 years and 5 months, according with previous studies that reported lower costs after 2 years of KT [24;29]. When assuming equal life expectancy for both modalities, KT is dominant over HD. The identification of patient subgroups categorized by baseline characteristics will help in the creation of reimbursement models adjusted to risk, preventing the issue of adverse selection. That would represent progress in efficiency and equity, the prime objectives of healthcare.
References
1. Thorp ML, Eastman L, Smith DH, Johnson ES. Managing the burden of chronic kidney disease. Dis Manag 2006;9(2):115-121. [ Links ]
2. Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 2005;20(12):2587-2593. [ Links ]
3. Carrilho MJ, Gonçalves C. Dinâmicas Territoriais do Envelhecimento: análise exploratória dos resultados dos Censos de 91 e 2001Available from: URL: www.censos.ine.pt/xportal/xmainxpid=CENSOS&xpgid=ine_censos_estudo_det&menuBOUI=13707294&contexto=es&ESTUDOSest_boui=106187&ESTUDOSmodo=2&selTab=tab1. [ Links ]
4. Nogueira ML. Custos com a diabetes. Boletim Sociedade Portuguesa de Diabetologia 2006; nº 4. [ Links ]
5. Macedo ME, Lima MJ, Silva AO, Alcantara P, Ramalhinho V, Carmona J. Prevalence, awareness, treatment and control of hypertension in Portugal: the PAP study. J Hypertens 2005;23(9):1661-1666. [ Links ]
6. Hagenmeyer EG, Häussler B, Hempel E, et al. Resource use and treatment costs after kidney transplantation: impact of demographic factors, comorbidities, and complications. Transplantation 2004;77(10):1545-1550. [ Links ]
7. Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and costeffectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess 2005 May;9(21):1-194. [ Links ]
8. Wit GA, Merkus MP, Krediet RT, de Charro FT. Health profiles and health preferences of dialysis patients. Nephrol Dial Transplant 2002;17(1):86-92. [ Links ]
9. Peeters P, Rublee D, Just PM, Joseph A. Analysis and interpretation of cost data in dialysis: review of Western European literature. Health Policy 2000;54(3):209-227. [ Links ]
10. Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 2002;22(5):417-430. [ Links ]
11. Machnicki G, Seriai L, Schnitzler M. Economics of transplantation: a review of the literature. Transplantation Reviews 2006 Apr 1;20(2):61-75. [ Links ]
12. Macário F. Tratamento substitutivo renal da doença renal crónica estadio V em Portugal. Relatório do Gabinete de Registo da Sociedade Portuguesa de Nefrologia. Available from: URL: www.spnefro.pt/comissoes_gabinetes/Gabinete_registo_2012/registo_2012.pdf. [ Links ]
13. Port FK, Bragg-Gresham JL, Metzger RA, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation 2002;74(9):1281-1286. [ Links ]
14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62-S69. [ Links ]
15. ERA-EDTA Registry. Annual Report 2003. www.era-edta-reg.org/indexjsp?p=annrep2006 [cited 2006 Mar 25]; Available from: URL: www.era-edta-reg.org/index.jsp?p=annrep [ Links ]
16. Rocha MJ, Ferreira S, Martins LS, et al. Cost analysis of renal replacement therapy by transplant in a system of bundled payment of dialysis. Clin Transplant 2012;26(4):529-531. [ Links ]
17. Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 2004;19(6):1594-1599. [ Links ]
18. Devine EB, Smith KL, Stehman-Breen C, Patrick DL. Health-related quality of life assessment in chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res 2003;3(1):89-100. [ Links ]
19. Cleemput I, Kesteloot K, De Geest S, Dobbels F, Vanrenterghem Y. Health professionals perceptions of health status after renal transplantation: a comparison with transplantation candidates expectations. Transplantation 2003;76(1):176-182. [ Links ]
20. Hathaway DK, Winsett RP, Johnson C, et al. Post kidney transplant quality of life prediction models. Clin Transplant 1998;12(3):168-174. [ Links ]
21. Matas AJ, Lawson W, McHugh L, et al. Employment patterns after successful kidney transplantation. Transplantation 1996;61(5):729-733. [ Links ]
22. Chaib-Eddour D, Chaib-Eddour H, Malaise J, Mourad M, Squifflet JP. Cost of renal transplant in Belgium. Transplant Proc 2005;37(6):2819-2820. [ Links ]
23. Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant 2011;26(9):2988-2995. [ Links ]
24. Villa G, Rodriguez-Carmona A, Fernandez-Ortiz L, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011;26(11):3709-3714. [ Links ]
25. Rodriguez DS, Jankowska-Gan E, Haynes LD, et al. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am J Transplant 2004;4(4):537-543. [ Links ]
26. Hunsicker LG. The economics of deceased donor transplantation: a microcosm of health care. Transplantation 2004;78(12):1711-1712. [ Links ]
27. Machado S, Figueiredo N, Neves M, et al. Kidney transplantation using donors over 70 years old: are the criteria for organ allocation too expanded? Transplant Proc 2012;44(8):2289-2292. [ Links ]
28. Portugal – Direcção-Geral da Saúde. Rede de Referenciação Hospitalar de Nefrologia. Lisboa: Direcção-Geral da Saúde. Direcção de Serviços de Planeamento; 2003. [ Links ]
29. Kaló Z. Economic aspects of renal transplantation. Transplant Proc 2003;35(3):1223-1226. [ Links ]
Drª Margarida Domingos
Hospital Curry Cabral, Department of Nephrology
Rua da Beneficência nº 8, 1069 – 166 Lisbon, Portugal.
E-mail: mbadomingos@gmail.com
Conflict of interest statement: None declared.
Received for publication: 04/07/2014
Accepted in revised form: 21/10/2014














