Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.1 Lisboa mar. 2015
CASE REPORT
Association of cyclosporine and mycophenolate mofetil in the treatment of steroid and cyclosporine resistant focal segmental glomerulosclerosis
Associação de ciclosporina e micofenolato de mofetil no tratamento de glomeruloesclerose segmentar e focal resistente à corticoterapia e ciclosporina
Marcio Viegas, Cristina Candido, Lucia Parreira, Jose Assuncao, Jose Vinhas
Nephrology Department, Centro Hospitalar de Setúbal. Setúbal, Portugal.
ABSTRACT
Background: Focal and segmental glomerulosclerosis is a common cause of nephrotic syndrome. In cases of steroid and cyclosporine resistance several alternatives have been suggested, based on limited studies with conflicting results. Several studies in organ transplantation have shown that the association of cyclosporine and mycophenolate mofetil has a synergic immunosuppressive effect. Clinical cases: We present two cases of nephrotic syndrome due to idiopathic focal segmental glomerulosclerosis with resistance to steroids, cyclosporine plus low -dose steroids and to mycophenolate mofetil in monotherapy in one of them, where complete remission was achieved with the association of cyclosporine and mycophenolate mofetil. There were no significant complications or renal failure during follow-up in both cases. Conclusion: Combined therapy with cyclosporine and mycophenolate mofetil appears to be effective and safe in the treatment of steroid and cyclosporine resistant idiopathic focal segmental glomerulosclerosis.
Key-Words: Cyclosporine; idiopathic focal and segmental glomerulosclerosis; mycophenolate mofetil.
RESUMO Background: A glomeruloesclerose segmentar e focal é uma causa comum de síndrome nefrótica. Em casos de resistência à corticoterapia e à ciclosporina várias alternativas têm sido sugeridas, com base em estudos limitados e com resultados discordantes. Vários estudos em transplante de órgãos têm demonstrado que a associação de ciclosporina e micofenolato de mofetil têm um efeito imunossupressor sinérgico. Casos clínicos: Apresentam -se dois casos de síndrome nefrótica devida a glomeruloesclerose segmentar e focal idiopática, com resistência à corticoterapia, a ciclosporina associada a baixa dose de corticoides e ao micofenolato de mofetil em monoterapia em um dos casos e onde a remissão completa foi alcançada com a associação de ciclosporina e micofenolato de mofetil. Ambos os casos não apresentaram complicações significativas durante o seguimento. Conclusão: A associação terapêutica de ciclosporina e micofenolato de mofetil parece ser eficaz e segura no tratamento de glomerulosclerose segmentar e focal idiopática resistente a corticoterapia e a ciclosporina.
Palavras-Chave: Ciclosporina; glomeruloesclerose segmentar e focal idiopática; micofenolato de mofetil.
BACKGROUND
Focal and segmental glomerulosclerosis (FSGS) is primarily a histological description, but also refers to a particular disease entity. FSGS is a common cause of nephrotic syndrome and can be secondary to multiple causes (mutations, viral infection, drugs, toxins or as the result of adaptive hyperfiltration injury) or idiopathic.
Antiproteinuric measures and hypertension control are essential for the management of both primary and secondary FSGS. In secondary FSGS, management is also based on correcting the specific cause.
As for idiopathic FSGS, in cases with nephrotic syndrome, treatment generally involves immunosuppressive therapy (steroids or calcineurin inhibitors in steroid-resistant FSGS)1. In situations of steroid and calcineurin inhibitors resistance many alternatives have been tested with discordant results. Mycophenolate mofetil (MMF), alone or in association with high -dose dexamethasone, is one of these alternatives but with conflicting response rates in several limited studies2-5. On the other hand, the synergic effect6,7 and safety6 of cyclosporine (CsA) and MMF association were already described. The authors describe two cases of nephrotic syndrome due to idiopathic FSGS with steroid and CsA resistance, successfully treated with CsA and MMF. The definitions of relapse, complete and partial remission were the same provided by KDIGO guidelines1.
CASE REPORTS Clinical and demographic aspects of the cases at presentation and histological description of renal biopsies are summarized in Table 1 and Table 2, respectively.
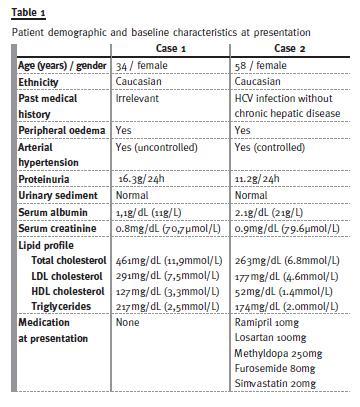
Case 1
After initial evaluation (clinical presentation described in Table 1), a renal biopsy (RB) was performed but had no renal fragment. The patient started treatment with enalapril 20mg, furosemide 40mg, simvastatin 20mg and steroids (prednisolone 1mg/Kg/day) empirically, but without clinical improvement after 4 months. At this stage, hospitalization and transitory ultrafiltration due to severe oedema were needed. Proteinuria and serum albumin were 20.0g/24h and 1.1g/dL (11g/L), respectively, with creatinine of 0.9mg/dL (79.6μmol/L). A second RB was performed and the result was interpreted as minimal change disease (biopsy characteristics described in Table 2) and treatment with CsA plus a low dose of prednisolone (0.15mg/Kg/day) was started, with serum CsA target between 125 and 175ng/mL (104–146 nmol/L), according to international guidelines1.
Despite an initial decrease of proteinuria to 4.1g/24h and a slight increase of serum albumin to 1.9g/dL (19g/L), after 4 months on this regimen there was progressive clinical worsening with generalized oedema, proteinuria (increase up to 20g/24h) and deterioration of renal function (serum creatinine elevation up to 2.5mg/dL / 221.24μmol/L). After 6 months, treatment with CsA plus low -dose of prednisolone was discontinued and a new RB was performed (Fig. 1a) that was compatible with FSGS (biopsy characteristics described in Table 2). After excluding secondary causes of FSGS, the patient was started on MMF (2000mg/day). Despite an initial decrease of proteinuria in the first 4 months down to 4.5g/24h, after this period, proteinuria increased again up to 22.5g/24h, despite progressive normalization of serum albumin and renal function. After 14 months of treatment with MMF without complete or partial remission, CsA (serum CsA target between 125 and 175 ng/mL / 104 – 146 nmol/L) was associated to the immunosuppressive regimen. The MMF dose was adjusted to 1000mg/day due to concern about potential risks associated with high immunosuppressive effect of this combination. After 6 months on this therapeutic regimen, complete remission was achieved and maintained during 8 months.
After this period (month 42) a relapse occurred, with progressive worsening of proteinuria and hypoalbuminemia, with serum creatinine below 1.0mg/dL (88.4 μmol/L), as described in a Fig. 2. Despite maintenance of nephrotic range proteinuria, clinical symptoms were minor, with only slight peripheral oedema until month 72. At this point, there was a significant worsening of the peripheral oedema and the dose of MMF was increased to 2000mg/day, maintaining the CsA dose.
Clinical improvement was rapidly obtained, with progressive improvement of proteinuria and serum albumin, achieving complete remission at month 84. After a very slow taper of CsA dose (currently 100mg/day), maintaining 2000mg/day of MMF, the patient remains in complete remission at 8.5 years of treatment, with no side-effects related to this immunosuppressive treatment and are normotensive without the need of antihypertensive drugs.
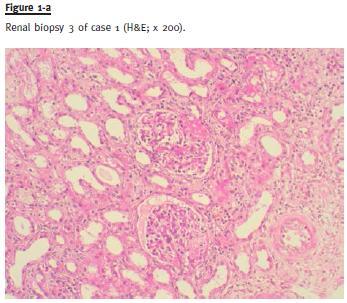
Case 2
After initial clinical evaluation (clinical presentation described in Table 2), an RB was performed that was compatible with FSGS tip lesion variant, described in Table 2 (Fig. 1-b). After hepatologist assessment to discard contraindications to immunosuppressive therapy, and exclusion of possible secondary aetiologies, treatment with steroids (prednisolone 1mg/Kg/day) was performed for 4 months without clinical improvement. Subsequently, the patient started CsA (serum CsA target between 125 and 175ng/mL / 104–146 nmol/L) plus a low dose of prednisolone (0.15mg/Kg/day). Under this therapeutic regimen, the patient maintained peripheral oedema and there was worsening of proteinuria up to 13.4g/24h. After 6 months without improvement, MMF (1000mg/day) was associated to the CsA and steroids were tapered and stopped. After 2 months of this immunosuppressive combination the oedema improved significantly, proteinuria decreased progressively and a serum albumin increase was achieved. Complete remission was reached after 3 years of treatment. With a follow -up of 3.5 years with slow taper of CsA (actually with 50mg/day) and maintaining the MMF dose, the patient maintains complete remission with serum creatinine of 1.0mg/dL (88.4 μmol/L), as shown in Fig. 2. Currently, the patient maintains losartan 100mg, atenolol 100mg and rilmenidine 2g daily for hypertension control. As in case 1, no side -effects related with this immunosuppressive treatment were detected during the follow-up period.
Clinical and demographic aspects of the cases at presentation and histological description of renal biopsies are summarized in Tables 1 and 2, respectively.
DISCUSSION
Both cases represent steroid and calcineurin inhibitors (CNIs) resistant idiopathic FSGS. In one of them there was resistance to MMF in monotherapy as well (an alternative regimen in cases of steroid resistant FSGS, if CNIs are not tolerated, but in association with high-dose dexamethasone1). In both cases, screening for secondary causes of FSGS was negative.
According to KDIGO guidelines, steroids are the first line treatment for idiopathic FSGS. In case of steroid resistance therapy with CsA plus a low dose of steroids is indicated1. Treatment of steroid and CNIs resistant FSGS is a challenge, with many therapeutic options proposed but with low levels of evidence to support their efficacy8. With or without steroids, MMF is one of these options, but the benefit of this immunosuppressive therapy is based only in observational studies [2 -5]. A few limited studies with tacrolimus suggest that this CNI may be efficient in cases of steroid and CsA resistant FSGS [9 -11]. There are also case reports that describe the use of rituximab in steroid resistant FSGS, with discordant results12,13. The use of adrenocorticotropic hormone (ACTH) in resistant nephrotic syndromes, including steroid resistant FSGS, was described in a few studies as a possible therapy in these cases14 -15.
Studies carried out in organ transplantation, showed that CsA and MMF combination has synergistic immunosuppressive effects16-18. Results of a study in kidney transplantation revealed similar calcineurin activity and cytokine inhibition with low-dose of CsA with MMF when compared with standard CsA dose in stable renal allografts. These results suggested that MMF could be synergistic with the pharmacodynamic effect of low dose CsA in maintenance immunosuppressive regimens18. Barten et al, observed an increase of maximal inhibition of T–cell surface antigens expression after combination drug therapy compared with MMF or CsA monotherapy, and a synergistic effect in cytokine production blockade as well19.
In both these clinical reports, CsA and MMF association had good results, with complete remission of nephrotic syndrome and normal renal function maintenance. However, only a few studies report this immunosuppressive combination in the treatment of steroid and CNI resistant FSGS, with poor results. Medrano et al., in a non-controlled study, tested the CsA (serum CsA target between 150 and 200ng/mL) and MMF (2000mg/day) association in the treatment of steroid and CsA resistant FSGS, with 4 out of 27 patients achieving partial remission. None achieved complete remission after 12 months of treatment20.
Nikibakhsh et al., in a paediatric study, reported two cases of complete remission in a subgroup of nine patients with steroid and CsA resistant FSGS after 6 months of treatment with this immunosuppressive association21.
Combined use of MMF and CSA was described in other glomerular diseases. Aragon et al. described this combination in paediatric proliferative lupus nephritis, with significant clinical and serological improvement, without significant adverse effects22.
The use of this therapeutic association, but with steroids in refractory nephrotic syndrome was also reported23.
Nephrotoxicity is one of the main issues of long-term use of CsA. Despite the long time of renal exposure to CsA, no repercussion in renal function was detected in both cases. This fact may be due to the low doses of CsA used after complete remission of nephrotic syndrome and possibly due to a MMF protective role in chronic nephrotoxicity of CsA.
In a retrospective study in liver transplant recipients, Karie -Guigues et al. showed that introduction of MMF without decreasing the CNI dose was associated wit h an improvement of renal function in the long term6.
Roos et al. showed that mycophenolic acid (active form of MMF) inhibited collagen expression and modified the migratory and functional properties of fibroblasts, thus directly exerting antifibrotic activities7.
Therefore, the favourable renal effects observed in CNIs minimization protocols used in organ transplant, that have been first only attributed to the lower CNIs exposure, may also result from a kidney protective effect of MMF. This apparent nephroprotective effect of MMF probably can also be applied to these two cases.
One important issue in these cases was therapy length until partial or complete remission. In the patients studied by the authors, in case 1, complete remission was obtain after 6 months of MMF and CsA, but in case 2 this goal was obtained only after 3 years of treatment. In these two cases, although different time until complete remission, clinical manifestations improved significantly after CsA and MMF were started. Another relevant question is the length of therapy since there are no clinical studies reporting long-term use of this therapeutic regimen for FSGS. Tapering and suspension were considered, but, due to the severity and refractory characteristics of the disease and since no complications of CsA and MMF association were observed in follow-up, this therapy was maintained with close monitoring for potential adverse effects.
CONCLUSION
According to these two clinical cases, combination of CsA and MMF appears to be an effective and safe treatment for steroid and cyclosporine resistant idiopathic FSGS. However, there are only a few studies using this immunosuppressive combination in the treatment of FSGS and, as seen above, with shorter length of treatment. Further studies with longer treatment length and larger number of patients are needed to confirm the efficacy, safety and optimal duration of this therapy in FSGS.
References
1. Group KDIGO. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter 2012; Suppl 2:139–274. [ Links ]
2. Choi MJ, Eustace JA, Gimenez LF, et al. Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int 2002; 61(3):1098 -1114. [ Links ]
3. Day CJ, Cockwell P, Lipkin GW, Savage CO, Howie AJ, Adu D. Mycophenolate mofetil in the treatment of resistant idiopathic nephrotic syndrome. Nephrol Dial Transplant 2002;17(11):2011 -2013 [ Links ]
4. Montané B, Abitbol C, Chandar J, Strauss J, Zilleruelo G. Novel therapy of focal glomerulosclerosis with mycophenolate and angiotensin blockade. Pediatr Nephrol 2003; 18(8):772 -777. [ Links ]
5. Cattran DC, Wang MM, Appel G, Matalon A, Briggs W. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin Nephrol 2004; 62(6):405-411. [ Links ]
6. Karie -Guigues S. Janus N, Saliba F, et al. Long-term renal function in liver transplant recipients and impact of immunosuppressive regimens (calcineurin inhibitors alone or in combination with mycophenolate mofetil): the TRY study. Liver Transpl. 2009; 15(9):1083–1091. [ Links ]
7. Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F. In vitro evidence for a direct anti -fibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther 2007;321(2):583–589. [ Links ]
8. Segarra -Medrano A, Jatem -Escalante E, Agraz -Pamplona I, et al. Treatment of idiopathic focal segmental glomerulosclerosis: options in the event of resistance to corticosteroids and calcineurin inhibitors. Nefrologia. 2013; 33(4):448 -461. [ Links ]
9. Segarra A, Vila J, Pou L, et al. Combined therapy of tacrolimus and corticosteroids in cyclosporin-resistant or-dependent idiopathic focal glomerulosclerosis: a preliminary uncontrolled study with prospective follow -up. Nephrol Dial Transplant 2002;17(4):655 -662. [ Links ]
10. McCauley J, Shapiro R, Ellis D, Igdal H, Tzakis A, Starzl TE. Pilot trial of FK 506 in the management of steroid -resistant nephrotic syndrome. Nephrol Dial Transplant 1993;8(11):1286 -1290. [ Links ]
11. Ramachandran R, Kumar V, Rathi M, et al. Tacrolimus therapy in adult-onset steroid--resistant nephrotic syndrome due to a focal segmental glomerulosclerosis single-center experience. Nephrol Dial Transplant 2014 Oct;29(10):1918 -1924. [ Links ]
12. Ochi A, Takei T, Nakayama K, et al. Rituximab treatment for adult patients with focal segmental glomerulosclerosis. Intern Med 2012;51(7):759-762. [ Links ]
13. Kronbichler A, König P, Busch M, Wolf G, Mayer G, Rudnicki M. Rituximab in adult patients with multi-relapsing/steroid -dependent minimal change disease and focal segmental glomerulosclerosis: a report of 5 cases. Wien Klin Wochenschr 2013;125(11 -12):328 -333. [ Links ]
14. Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol 2012;36(1):58 -67. [ Links ]
15. Hogan J, Bomback AS, Mehta K, et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol 2013;8(12):2072 -2081. [ Links ]
18. Beckebaum S, Cicinnati VR. Conversion to combined mycophenolate mofetil and low -dose calcineurin inhibitor therapy for renal dysfunction in liver transplant patients: never too late? Dig Dis Sci 2011; 56(1):4–6. [ Links ]
19. Farkas SA, Schnitzbauer AA, Kirchner G, Obed A, Banas B, Schlitt HJ. Calcineurin inhibitor minimization protocols in liver transplantation. Transpl Int 2009;22(1):49-60. [ Links ]
20. Grinyó JM, Cruzado JM, Millán O, et al. Low -dose cyclosporine with mycophenolate mofetil induces similar calcineurin activity and cytokine inhibition as does standard-dose cyclosporine in stable renal allografts. Transplantation 2004; 78(9):1400 -1403. [ Links ]
21. Barten MJ, Dhein S, Chang H, et al. Assessment of immunosuppressive drug interactions: inhibition of lymphocyte function in peripheral human blood. J Immunol Methods 2003;283(1 -2):99 -114. [ Links ]
22. Segarra Medrano A, Vila Presas J, Pou Clavé L, Majó Masferrer J, Camps Doménech J. Efficacy and safety of combined cyclosporin A and mycophenolate mofetil therapy in patients with cyclosporin -resistant focal segmental glomerulosclerosis. Nefrologia. 2011; 31(3):286-291.
23. Nikibakhsh AA, Mahmoodzadeh H, Karamyyar M, Hejazi S, Noroozi M, Macooie AA. Treatment of steroid and cyclosporine -resistant idiopathic nephrotic syndrome in children. Int J Nephrol 2011;2011:930965. [ Links ]
24. Aragon E, Chan YH, Ng KH, Lau YW, Tan PH, Yap HK. Good outcomes with mycophenolate-cyclosporine-based induction protocol in children with severe proliferative lupus nephritis. Lupus 2010;19(8):965 -973. [ Links ]
25. Jang HR, Jung HW, Lee YJ, et al. Combination treatment with corticosteroid, cyclosporine A, and mycophenolate in refractory nephrotic syndrome. Clin Nephrol 2011;75(6):511 -517. [ Links ]
Dr. Márcio Viegas
Department of Nephrology, Hospital de São Bernardo
Rua Camilo Castelo Branco, 2910 – 446 Setúbal, Portugal.
E -mail: marciosrviegas@gmail.com
Conflict of interest statement: None declared.
Received for publication: 12/10/2014
Accepted in revised form: 09/01/2015














