Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.3 Lisboa set. 2015
ORIGINAL ARTICLE
Fgf-23 and vascular calcification in a peritoneal dialysis population with residual renal function
Fgf-23 e calcificação vascular numa população em diálise peritoneal com função renal residual
Sofia Santos1, José Carlos Oliveira2, Tiago Barra3, Andreia Campos1, Maria João Carvalho4, Jorge Malheiro4, Isabel Fonseca4, António Cabrita1, Teresa Adragão5, Anabela Rodrigues4
1 Nephrology Department, Centro Hospitalar do Porto, Porto, Portugal.
2 Clinical Chemistry Department, Centro Hospitalar do Porto, Porto, Portugal.
3 Nephrology Department, Hospital de São Teotónio, Viseu, Portugal.
4 Nephrology Department, Centro Hospitalar do Porto / UMIB, Porto, Portugal.
5 Nephrology Department, Hospital Santa Cruz, Lisboa, Portugal.
ABSTRACT
Introduction and Aims: Fibroblast growth factor 23 (FGF-23) induces phosphaturia. Its clinical impact is beyond mineral bone disease in chronic kidney disease (CKD), being coupled with vascular calcification and mortality. Residual renal function (RRF) is associated with significant capacity to excrete phosphate in peri- toneal dialysis (PD). Besides testing whether FGF-23 is still related with glomerular filtration rate (GFR) and phosphate excretion in this late stage of CKD (5d), we aimed to explore its link with vascular calcification. Subjects and Methods: FGF-23 (C terminal) was measured in forty prevalent PD patients with RRF, aged 61.5 (51.0-67.0) years old, in renal replacement therapy (RRT) for 43.5 (23-80.0) months; 36.6% were female, 19.5% had diabetes mellitus and 37.5% were under automated PD regimen; 80% were on PD first, and only 20% had previous RRT. Relevant variables including dietary phosphate (P) intake, CKD-bone laboratory parameters, serum 25-hydroxyvitamin D, magnesium (Mg) levels, GFR, urinary phosphate, fractional excretion of phosphorus (FEP), albumin, proBNP and Adragão vascular calcification score were explored. Results: Median levels (25-75% range) of serum variables were: FGF-23 1997 (1623-2149) RU/mL, Mg 0.94 (0.8-1.0) mmol/L, 25-hydroxyvitamin D 30 (18-47) nmol/L, calcium 2.2 (2.0-2.37) mmol/L, phosphorus 1.69 (1.30-1.90) mmol/L, PTH 429 (309-626) pg/mL. FGF-23 correlated positively with serum phosphate (r = 0.39, p = 0.013) and negatively with urine volume (r = -0.48, p = 0.001), phosphaturia (r = -0.594, p < 0.0001) and GFR (r =-0.61, p < 0.0001). However, FGF-23 was not significantly correlated with age, total time of RRT, dietary P, FEP, Mg, nor 25-hydroxyvitamin D. High FGF-23 group had higher FEP. GFR was the single inde- pendent predictor of increased FGF-23. On the other hand, neither FGF-23 nor low FEP/FGF-23 ratio were significantly associated with the vascular calcification score. Only albumin (lower), magnesium (lower) and proBNP (higher) levels significantly differed in calcified versus non-calcified patients (all with p < 0.05). Conclusions: In our population, FGF-23 was not associated with vascular calcification. GFR was the single independent predictor of increased FGF-23 in patients with diuresis. Increment of FGF-23 in PD patients signalizes an active endocrine phosphaturic process compensating renal function loss, as expressed by higher fractional excretion of phosphorus. It alerts for dietetic and therapy optimization. However, its link with vascular calcification still lacks validation.
Key-Words: FGF-23; fractional excretion of phosphorus; peritoneal dialysis; phosphaturia; residual renal function; vascular calcification.
RESUMO
Introdução: O fator de crescimento do fibroblasto 23 (FGF-23) induz fosfatúria. A sua importância clínica ultrapassa o seu impacto na doença óssea da doença renal, associando-se, segundo alguns autores, à calcificação vascular e à mortalidade. Nos doentes em diálise peritoneal (DP), a função renal residual (FRR) é responsável por uma fracção significativa da excreção do fósforo. O nosso objectivo foi testar se o FGF -23 se relaciona com o débito do filtrado glomerular (DFG) e a excreção de fosfato nesta fase tardia da DRC(5d), focando a sua relação com calcificação vascular. Métodos: O FGF-23 (C terminal) foi medido em 40 doentes prevalentes em DP com função renal residual, com idade de 61.5 (51,0-67,0) anos, em tratamento substitutivo da função renal há 43,5 (23-80,0) meses. Destes doentes, 36,6% eram do sexo feminino 19,5 % tinham diabetes mellitus e 37,5% estavam sob regime de DP automática; 80% iniciaram diálise com DP; apenas 20% estiveram previamente sob outra técnica substitutiva da função renal. Foram exploradas variáveis clínicas relevantes, nomeadamente fosfato dietético, parâmetros ósseos, 25-hidroxivitamina D, magnésio (Mg), DFG, fosfatúria, fração excrecional de fosfato (FEP), albumina, proBNP e o score de calcificação vascular de Adragão. Resultados: Os valores medianos (IQ 25-75) das variáveis séricas foram: FGF-23 1997 (1623-2149) RU/ mL, Mg 0,9 (0,8-1,0) mmol/L, 25-hidroxivitamina D 30 (18-47) nmol/L, cálcio 2,2 (2,0-2,4) mmol/L, fósforo 1,7 (1,3-1–,9) mmol/L, PTH 429 (309-626) pg/mL. O FGF-23 correlacionou-se positivamente com o fósforo sérico (r = 0,39, p = 0,013) e negativamente com o volume de urina (r = -0,48, p = 0,001), fosfatúria (r = -0,594, p < 0,0001) e DFG (r = -0,61, p < 0,0001). No entanto, não se verificou qualquer relação significativa com idade, tempo em terapêutica da substituição da função renal, fósforo dietético, FEP, Mg ou 25-hidroxivitamina D. O grupo com valor elevado de FGF-23 apresentava aumento da FEP. O DFG foi preditor independente de aumento do FGF-23. Por outro lado, nem o FGF-23 nem o rácio baixo FEP/FGF-23 se associaram significati- vamente com o score de calcificação vascular. A albumina (baixa), o magnésio (baixo) e o proBNP (elevado) foram significativamente diferentes em doentes calcificados quando comparados com não calcificados (todos com p < 0,05). Conclusões: No nosso estudo não foi confirmada a associação do FGF-23 com a calcificação vascular. O DFG foi o único preditor independente do aumento de FGF-23 em doentes sob diálise peritoneal com função renal residual. O aumento do FGF-23 sinaliza um processo fosfatúrico ativo, compensatório face à perda de FRR, tal como é expresso pelo aumento da fracção excrecional de fósforo. Poderá alertar para oportuna optimização da dieta e da terapêutica no âmbito do metabolismo fosfocálcico. No entanto a sua associação com calcificação vascular permanece por validar.
Palavras-Chave: Calcificação vascular; diálise peritoneal; FGF-23; fosfatúria; fração excrecional de fósforo; função renal residual.
INTRODUCTION
Fibroblast growth factor -23 (FGF-23) is a hormone whose main known function is the regulation of the phosphate concentration in plasma. It is secreted by osteocytes and acts on the kidneys, at the FGF receptor (FGFR)-klotho complex, to increase the excretion of phosphate. The FGF-23 also inhibits the conversion of calcidiol (25[OH] vitamin D) to calcitriol (1.25[OH]2 vitamin D)1. According with these actions, FGF-23 induces phosphaturia and is responsible for a sig- nificant part of phosphate metabolism and control2. In CKD, as urinary phosphate excretion declines FGF-23 levels increase and can reach 1000-fold above normal in end stage renal disease (ESRD)3. In recent years, an association was reported between FGF-23 and all-cause mortality, not only in people undergo- ing dialysis4, but also in pre-dialysis patients5.
Hyperphosphatemia often occurs in dialysis patients and is an independent risk factor for arterial calcification, associated with cardiovascular events and mortality6. Residual renal function (RRF) is responsible for a significant capacity to excrete phosphate, therefore, FGF23 seems to be a key mediator of serum phosphorus levels control in patients with diuresis. However, the role for FGF-23 in the process of progressive arterial calcification is not clear7.
Since the clinical impact of high FGF-23 appears to be beyond mineral bone disease in CKD, its measurement may identify a different dimension of kidney function that is not fully captured by glomerular filtration rate. Therefore, besides testing whether FGF-23 is still related with GFR and phosphate excretion in this late stage of CKD (5d), we aimed to explore its link with vascular calcification.
SUBJECTS AND METHODS
Study population:
We have studied 40 prevalent and stable patients aged 18 years or older who had been undergoing chronic peritoneal dialysis (continuous ambulatory peritoneal dialysis or continuous cyclic peritoneal dialysis) at the division of Nephrology at Centro Hospitalar do Porto, Portugal.
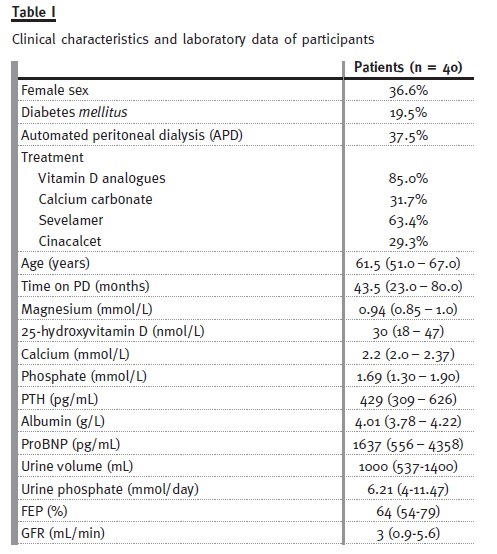
Inclusion criteria: To be included in the study the patients had to be on PD for at least 3 months and have residual renal function (as measured by diuresis > 100mL/day). The clinical characteristics and laboratory data of our 40 patients treated with peritoneal dialysis are summarized in Table I.
Data collection and measurements:
We have analysed the following data that was collected at a single random visit to the peritoneal dialysis centre: demographic characteristics, medical history, smoking status, use of active vitamin D analogues, cinacalcet or oral phosphate binders, dose of dialysis, GFR, and blood and urinary samples. Comprehensive metabolic panels and urinary biochemical panels were measured using standard assays. A dietary inquiry was done and the ingested daily amount of phosphate was calculated. The peritoneal clearance of phosphate was measured.
Urinary fractional excretion of phosphate (FEP) was calculated as follows: [urine phosphorus (mg/ dl)/serum phosphorus (mg/dl)] × [serum creatinine (mg/dl)/urine creatinine (mg/dl)] × 100.
Plasma FGF-23 was measured using the second generation C-terminal assay. Samples were immediately centrifuged, aliquoted, and stored at -80 oC until batched assays were performed. We have also applied the Adragão vascular calcification score8 at 32 patients after excluding those who missed appointment for radiological testing. Adragão et al.8 showed that vascular calcification score ≥3 is the best cut-off value associated with cardiovascular mortality and cardiovascular events, reason why we used that value to dictate a positive vascular score. All tests and radiographic films of pelvis and hands were performed and blindly classified with Adragão score by a single investigator, at Centro Hospitalar do Porto, Portugal.
Statistical analysis:
We used standard descriptive statistics to assess clinical and laboratory data at baseline. Continuous variables were summarized as median and interquartile range (IQR). Categorical variables were expressed as frequencies and proportions. All variables were explored cross-sectionally. Pearson or Spearmen correlations were used as appropriate. Mann-Whitney U test was used to investigate variables in subgroups.
The FGF-23 variable was categorized as FGF-23 tertile (below or above 1800 RU/ml) and investigated by multivariable logistic regression. For all comparisons, a p-value < 0.05 was considered statistically significant.
RESULTS
Associations of demographic, clinical, and laboratory characteristics with FGF-23:
The median concentration and IQ25-75 of immu- noreactive FGF-23 was 1997 (1623 – 2149) reference units (RU/ml).
The FGF-23 correlated positively with serum phosphate (r = 0.39, p = 0.011) and negatively with urine volume (r = -0.48, p = 0.002), phosphaturia (r = -0.594, p < 0.0001) and GFR (r =- 0.57, p < 0.0001). However FGF-23 was not significantly correlated with age, time on dialysis, dietary phosphate ingestion, FEP, Mg, nor 25-hydroxyvitamin D.
We present the variables in subgroups categorized according to FGF-23 30% tertile (Table II).
The high FGF-23 levels group presented significantly lower GFR, higher serum phosphate and higher urinary fractional excretion of phosphate (Fig. 1).
Multivariable logistic regression showed that GFR was the sole independent predictor of FGF-23. Each 1 ml/min decrease in GFR was associated with a 90% chance of having higher FGF-23 (OR 1.898 (1.226-2.939), p = 0.004).
FGF-23 and arterial calcification
Radial and digital calcifications were present in 19 patients (59.4%) and iliac and femoral calcifications were present in 21 patients (65.6%).
The distribution of vascular calcification score in the 32 patients was the following: score 0 in nine patients (28.1%), score 1 in one patient (3.1%), score 2 in four patients (12.5%), score 3 in seven patients (22.0%), score 4 in five patients (15.6%), score 5 in one patient (3.1%), score 6 in three patients (9.4%) and score 7 and 8 both in one patient (each 3.1%).
The comparison between patients with positive vascular calcification score (score ≥3) versus low calcified patients showed that FGF-23 and low FEP/ FGF-23 were not significantly associated with vascu- lar calcification.
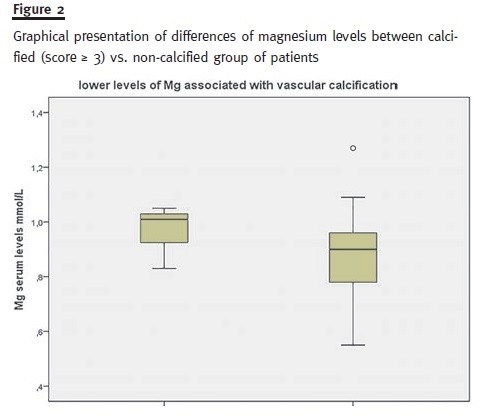
By exploring subgroups with the Mann-Whitney U test only albumin (lower levels, p = 0.046), pro- BNP (higher levels, p = 0.005) and magnesium (lower levels, p = 0.026) significantly differed between calcified (score ≥ 3) versus non-calcified group of patients (Fig. 2).
DISCUSSION
The results of this cross-sectional study confirm that FGF-23 levels are markedly elevated in patients with ESRD and are associated with hyperphosphatemia, but were not associated with vascular calcification. Previous studies sustain the use of FGF-23 as a stable biomarker of disordered phosphate metabolism9. Our study also showed that lower GFR determine higher FGF-23 levels. Indeed the amount of FGF-23 increases dramatically in dialysis patients. Data on FGF-23 levels in patients undergoing PD is limited. Isakova et al.9 showed that among PD adults with residual renal function, there is a continuous relationship between higher FGF-23 and lower residual renal function. However, we did not find any association between dialysis vintage and higher FGF-23. The limited number of patients that had previous RRT comparing to those on PD first (80%) did not allow to reliably test if FGF23 is significantly increased in patients with previous haemodialysis course of time.
There is still a lack of understanding on the determinants of FGF-23 serum concentrations. This issue was recently revised by the Frazão group10 and dietary phosphate has been pointed as a putative predictor. We were unable to document a significant difference in ingested phosphate load between the groups, but our population had high phosphate ingestion (1000-1400 mg/day), above the recommended target of 800-1000 mg/day, effacing the oppor- tunity to test this hypothesis. This, however, remains a relevant issue in a population of dialysis patients prone to progressively loose renal capability to excrete ingested phosphate load. However, the phosphaturic action of FGF-23 is signalized by the higher phosphate fractional excretion present in the high FGF-23 group.
In early CKD progression FGF-23 is elevated well before serum phosphate levels are increased11 and is not correlated with PTH. The FGF-23 also presents a more stable laboratory profile and less intra-individual variability than PTH12 being an opportune tool to signalize the patient who may benefit from dietary counseling, dialysis optimization or higher dose of phosphate chelating agents.
We have also showed that therapy was adequately adjusted to increase dialysis solute removal since peritoneal phosphate clearance was higher in patients with lower renal function. Additionally, we tested the link between FGF-23 and vascular calcifi- cation. A low fractional excretion of phosphate/ FGF-23 ratio has been associated with severe abdominal aortic calcification in stage 3 and 4 CKD13. But, in our population FGF-23 or low fractional excretion of phosphate/FGF-23 ratio were not significantly associated with a positive vascular calcification score: the modest sample of patients may have limited the ability to demonstrate significant differences between calcified versus non-calcified groups. However, the results published in literature for vascular calcification also remain conflicting7, 14.
Interestingly our results suggest an association of hypomagnesemia and vascular calcification, as previously documented by other investigators15 pointing that hypomagnesemia and high FGF-23 levels were independent predictors of mitral valve calcification and intima-media-thickness in 150 diabetic patients with mild to moderate CKD. It is stated that magnesium antagonizes phosphate-induced apoptosis of vascular smooth muscle cells preven- ting vascular calcification and it was shown that magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease16. The cellular pathways of magnesium interference with vascular calcification are presently under investigation17.
We acknowledge some limitations in our study, particularly dependent on the small sample of enrolled patients and the cross-sectional design. Methodological skills also need to be optimized since the measurement of FGF-23 is still investigational: the reagents in 2nd generation human FGF-23 ELISA kit have been optimized, so that the high dose hook effect is not a problem for samples with elevated FGF-23 values. Samples with levels between the highest standard and 750,000 RU/mL will read greater than the highest standard and were diluted 1:10 or greater with the 0 RU/mL calibrator. But dilution of high-reading samples with saline may result in significant deviation from linearity. Besides current ELISA kits for plasma intact FGF-23 measurement show poor analytical agreement and cannot be used interchangeably18.
There is a scarce number of studies addressing the pathophysiological axis of FGF-23 in peritoneal dialysis, therefore, it seems to us that the present study is opportune and points to investigational tracks such as the link between magnesium and vascular calcification. Longitudinal design will be mandatory to clarify other putative determinants of FGF-23 and its ultimate clinical impact on PD patients.
CONCLUSIONS
Fibroblast growth factor 23 was not associated with vascular calcification in our population. Glomerular filtration rate was the single independent predictor of increased FGF-23 on PD patients with significant RRF. Increment of FGF-23 in PD patients signalizes an active endocrine phosphaturic process compensating renal function loss as expressed by higher fractional excretion of phosphorus. It alerts for dietetic and therapy optimization. Howe- ver, its link with vascular calcification still lacks validation.
References
1. Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol 2007;18(6):1637-1647. [ Links ]
2. Fukumoto S. Physiological regulation and disorders of phosphate metabolism- piv- otal role of fibroblast growth factor 23. Intern Med 2008;47(5):337-343. [ Links ]
3. Isakova T. Fibroblast growth factor 23 and adverse clinical outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 2012;21(3):334-340. [ Links ]
4. Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359(6):584-592. [ Links ]
5. Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011;22(10):1913-1922.
6. Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009;75(9):890-897. [ Links ]
7. Scialla JJ, Lau WL, Reilly MP, et al. with the Chronic Renal Insufficiency Cohort Study Investigators. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 2013;83(6):1159-1168. [ Links ]
8. Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 2004;19(6):1480- 1488. [ Links ]
9. Isakova T, Xie H, Barchi-Chung A, et al. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6(11):2688-2695. [ Links ]
10. Diniz H, Frazão JM. The role of fibroblast growth factor 23 in chronic kidney disease-mineral and bone disorder. Nefrologia 2013;33(6):835-844. [ Links ]
11. Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011;22(5):956-966.
12.Jia T, Qureshi AR, Brandenburg V, et al. Determinants of fibroblast growth factor-23 and parathyroid hormone variability in dialysis patients. Am J Nephrol 2013;37(5):462-471. [ Links ]
13. Craver L, Dusso A, Martinez-Alonso M, Sarro F, Valdivielso JM, Fernández E. A low fractional excretion of Phosphate/FGF23 ratio is associated with severe abdominal aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol 2013;14:221. [ Links ]
14. Desjardins L, Liabeuf S, Renard C, et al. with the European Uremic Toxin (EUTox) Work Group. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporosis Int 2012;23(7):2017- 2025. [ Links ]
15. Silva AP, Gundlach K, Buchel J, et al. Low magnesium levels and FGF-23 dysregulation predict mitral valve calcification as well as intima media thickness in predialysis diabetic patients. Int J Endocrinol doi: 10.1155/2015/308190. [ Links ]
16. Sakaguchi Y, Iwatani H, Hamano T, et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int doi: 10.1038/ki.2015.165. [ Links ]
17. Louvet L, Bazin D, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PloS One 2015;10(1):e0115342. [ Links ]
18. Smith ER, McMahon LP, Holt SG. Method-specific differences in plasma fibroblast growth factor 23 measurement using four commercial ELISAs. Clin Chem Lab Med 2013;51(10):1971-1981. [ Links ]
Dra Sofia Santos
Department of Nephrology
Centro Hospitalar do Porto – Hospital Geral de Santo António
Largo Prof. Abel Salazar 4099-001 Porto, Portugal.
E-mail: sofia.fersantos@gmail.com
Conflicts of interest statement: None declared.
Received for publication: 14/07/2015
Accepted in revised form: 08/09/2015














