Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.4 Lisboa dez. 2015
ORIGINAL ARTICLE
Magnesium – association with inflammation and renal disease in systemic lupus erythematosus
Magnésio – associação com inflamação e doença renal no lúpus eritematoso sistémico
Liliana Cunha1, Gisela Marcelino2, Marta C. Amaral3,4, J. Delgado Alves3,4
1 Department of Nephrology Hospital Prof. Dr. Fernando Fonseca, Amadora, Portugal.
2 Internal Medicine and Rehabilitation Service, Trois-Chêne Hospital, Geneva University Hospitals, Geneva, Switzerland.
3 Department of Medicine IV, Hospital Prof. Dr. Fernando Fonseca, Unidade de Doencas Imunomediadas Sistémicas (UDIMS), Amadora, Portugal.
4 CEDOC – Chronic Diseases Research Centre, NOVA Medical School/Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Lisboa, Portugal
ABSTRACT
Introduction: Recent studies suggest that magnesium deficiency may play a role in inflammation. In diabetes and cardio-vascular diseases, conditions with a component of chronic inflammation, C–reactive protein levels are higher and associated with low serum magnesium. The objective of this study is to evaluate serum magnesium levels in patients with systemic lupus erythematosus and its potential association with inflammation and renal manifestations. Methods: All patients with systemic lupus erythematosus followed in a Systemic Immune Diseases Unit, from January 2012 until January 2014, were included in this cross sectional analysis.
Patients with infection, neoplasia, liver failure and chronic kidney disease (stage > 3) were excluded. Clinical information and laboratory results (serum magnesium, C-reactive protein, erythrocyte sedimentation rate, serum creatinine and spot urine test) were collected. A multivariate analysis was performed to explore possible predictive factors for hypomagnesaemia. Results: One hundred and two patients were included (94.1% female, 21-86 years). 33.4% had hypertension, 8.8% had diabetes and 20.6% had hypomagnesaemia (< 1.8mg/dL). There were no significant differences between the inflammatory parameters of patients with hypomagnesaemia or normomagnesaemia.
Serum magnesium was significantly lower with increasing comorbidities (p = 0.01). Leukocyturia was significantly higher in the hypomagnesaemia group (p = 0.03) and haematuria had a negative correlation with serum magnesium (-0.23, p < 0.05). Multivariate analysis showed that patients with hypertension and diabetes had higher risk of hypomagnesaemia: OR 42.29 (95% CI, 1.43-1249.31). Leukocyturia was also individually and independently associated with hypomagnesaemia: OR 8.37 (95% CI, 1.40-49.97). Conclusion: The presence of hypomagnesaemia in our patients with systemic lupus erythematosus was high. There was no association between the levels of serum magnesium and the inflammatory parameters. Increasing comorbidities and leukocyturia were independent predictors of lower serum magnesium. Finally, the association of leukocyturia and haematuria with lower serum magnesium may suggest a relationship with a higher disease activity.
Key-Words: Inflammation; magnesium deficiency; systemic lupus erythematosus.
RESUMO
Introdução: Estudos recentes sugerem uma relação entre inflamação e défice de magnésio. Em doenças com inflamação crónica, como a diabetes e doenças cárdio-vasculares, os níveis séricos de proteína C-reativa são mais altos e associados a menores níveis séricos de magnésio. O objetivo desde trabalho é avaliar o magnésio sérico em doentes com lúpus eritematoso sistémico e a sua associação com inflamação e manifestações renais. Métodos: Foram incluídos neste estudo transversal, doentes com lúpus eritematoso sistémico, seguidos numa unidade de doenças imunomediadas sistémicas, entre Janeiro 2012 e Janeiro 2014.
Foram excluídos os doentes com infeção, neoplasia, insuficiência hepática e doença renal crónica (estádio > 3). Foi colhida informação clínica e laboratorial (magnésio sérico, proteína C-reativa, velocidade de sedimentação, creatinina sérica e exame sumário de urina). Foi usada uma análise multivariada para explorar possíveis fatores preditivos de hipomagnesémia. Resultados: Foram incluídos 102 doentes (94.1% do sexo feminino, 21-86 anos), 33.4% com hipertensão, 8.8% com diabetes e 20.6% com hipomagnesémia (< 1.8mg/dL). Não houve diferenças significativas nos parâmetros inflamatórios entre os doentes com hipomagnesémia e normomagnesémia. Os valores de magnésio foram mais baixos nos doentes com mais comorbilidades associadas (p = 0.01). A leucocitúria foi significativamente maior no grupo com hipomagnesémia (p = 0.03) e a hematúria correlacionou-se negativamente com o magnésio sérico (r = -0.23, p < 0.05). Na análise multivariada, doentes com hipertensão e diabetes apresentaram maior risco de hipomagnesemia: OR 42.29 (95% CI, 1.43-1249.31). A leucocitúria foi individualmente e independentemente associada a hipomagnesemia OR 8.37 (95% CI, 1.40-49.97). Conclusão: A presença de hipomagnesémia nos nossos doentes com lúpus eritematoso sistémico foi elevada. Não encontrámos associação entre os níveis de magnésio sérico e os parâmetros inflamatórios. A comorbilidade crescente e a leucocitúria foram preditores independentes de hipomagnesémia. A associação de leucocitúria e hematúria com magnésio sérico mais baixo pode sugerir uma relação com maior atividade da doença.
Palavras-Chave: Deficiência magnésio; inflamação; lúpus eritematoso sistémico.
INTRODUCTION
Experimental evidence suggests that magnesium deficiency may play a role in inflammation, although its specific interference with the inflammatory response is not fully elucidated1-3.
Magnesium has several actions that could explain its anti-inflammatory activity: it is a mild physiologic calcium blocker that decreases intracellular calcium level, blocks sodium attachment to vascular smooth muscle cells, increases the vasodilator prostaglandin E1, increases nitric oxide, reduces angiotensin-induced aldosterone synthesis, reduces triglycerides and increases high-density lipoprotein, through an increase in lipoprotein lipase activity and by inhibiting HMGCoA reductase. Most of these cardiovascular and oxidative effects could explain the higher inflammatory state seen in states of magnesium deficiency1-4.
In animal models, magnesium deficiency leads to pro-inflammatory responses characterised by elevations of C-reactive protein (CRP) and nuclear factor kappa B, by leukocyte and macrophage activation, platelet aggregation and endothelial proliferation; all due to up-regulation of pro-inflammatory cytokines1,4,5.
Magnesium deficiency is present in 13.5 to 47.7% of patients with type 2 diabetes and is associated with the development of insulin resistance and inadequate metabolic control6-9. In addition, patients with diabetes have higher levels of CRP reflecting higher levels of chronic inflammation10. The association of hypomagnesaemia with chronic inflammatory diseases has also been described in metabolic syndrome, hypertension, atherosclerosis and cardiovascular disease11-13. Deficient magnesium intake and low serum magnesium levels have been associated with elevated CRP, a widely used indicator of inflammation14,15.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with multiorgan inflammation, characterized by a global loss of self-tolerance with activation of autoreactive T and B cells, that leads to the production of pathogenic autoantibodies and tissue injury16. Elevated CRP (although at low levels) and raised erythrocyte sedimentation rate (ESR) are usually associated with clinically active disease measured by the SLEDAI score17,18. SLE patients with elevation of inflammatory markers (CRP and ESR) are also at higher risk of death19.
Magnesium deficiency has been described in patients with SLE but the association to inflammation has not been studied20. In SLE, a chronic inflammatory disease, low serum magnesium levels may be linked to inflammation. Low serum magnesium could also be associated with greater disease activity, particularly with renal manifestations.
The objective of this study is to evaluate the association of serum magnesium levels with thedegree of inflammation (as evaluated by CRP and ESR) and renal manifestations [evaluated with estimated glomerular filtration rate (eGFR) and urinalysis] in patients with SLE.
SUBJECTS AND METHODS
Patients fulfilling the American College of Rheumatology criteria for SLE21, followed in the Systemic Immune-mediated Diseases Unit at our Hospital, from January 2012 until January 2014, were included in this cross sectional analysis. The patients were evaluated by the attending physician in the outpatient clinic and blood sample analyses were performed up to one month before. The blood sample analysis elected for each patient included at least the determination of serum magnesium, ESR and CRP (giving preference to the set of values with lower CRP).
When it was not evaluated in the same analysis, the serum creatinine (SCr) value was included with a maximum interval of one month. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula22. Patients with concomitant infection, neoplasia, liver failure and chronic kidney disease (CKD) in stages 4 and 5 according to K/DOQI 2002 classification (eGFR < 30mL/min/1.73m2)23 were excluded.
Clinical information collected included: age, gender, presence of hypertension and diabetes, and current therapy [including steroids, other immunosuppressive drugs, diuretics and proton pump inhibitors (PPI)]. Laboratory results included: serum magnesium mg/dL, CRP mg/dL, ESR mm/h, SCr mg/dL and a spot urine test (when available).
The spot urine test is a semi-quantitative analysis that includes the following ranges: proteinuria – negative, 50mg/dL, 100mg/dL, 200 mg/dL, 300 mg/dL, 400 mg/dL; haematuria (quantification of heme concentration) – negative, 1+ (0.06mg/dL), 2+ (0.2mg/ dL) and 3+ (1mg/dL); leukocyturia (quantification of leukocyte esterase concentration) – negative, 25 UI/L, 75 UI/L, 250 UI/L, 500 UI/L.
Hypomagnesaemia was defined as serum magnesium less than 1.8 mg/dL and patients were divided in two groups accordingly: hypomagnesaemia and normomagnesaemia. Patients were also divided according to the presence of comorbidities: without hypertension and diabetes, with hypertension and with hypertension and diabetes.
The CRP and ESR values were considered indirect indicators of inflammation and renal disease was evaluated with eGFR, proteinuria, haematuria and leukocyturia.
Statistical analysis was performed using IBM SPSS Statistics 20 for Windows. Comparisons between groups were performed using chi-square for qualitative variables and Mann-Whitney and Kruskal-Wallis tests for non-parametric continuous variables. To explore the association between serum magnesium and the different variables, the Spearman correlation factor was calculated. A multivariate analysis was also performed to explore possible predictive factors of low serum magnesium, controlling for the potential confounders. The results were considered significant if p < 0.05.
RESULTS
One hundred and two patients were included (94.1% women), with ages ranging from 21 to 86 years. Hypertension was present in 33.4%, diabetes in 8.8% and CKD stage 3 in 14.7% of all patients.
Hypomagnesaemia was present in 20.6% of all patients and in 15.2% of patients without hypertension or diabetes.
There were no significant differences between the hypomagnesaemia and normomagnesaemia groups concerning age, diabetes, eGFR, CKD stage 3, type of immunosuppressive therapy, diuretic or PPI use.
There were no differences regarding ESR or CRP levels (Table I).
There was a significant difference between the two groups in relation to hypertension, with the hypomagnesaemia group having a higher percentage of patients with hypertension (52.4% versus 27.2%, p = 0.03). The same trend was observed between groups concerning diabetes (p = 0.06), (Table I).
In addition, 44.4% of patients with diabetes and 66.7% of patients with hypertension and diabetes had hypomagnesaemia. Leukocyturia was significantly higher in the hypomagnesaemia group (p = 0.03), (Table I).
When patients were classified according to the presence of comorbidities, C-reactive protein was significantly higher and serum magnesium was significantly lower in the group with more comorbidities (p = 0.003 and p = 0.01, respectively) (Table II).
In addition, the prednisolone dose was significantly higher in the hypertension+diabetes group (p = 0.04). Concerning renal function, eGFR was significantly lower with increased comorbidities (p < 0.001).
There were no significant differences between groups concerning ESR, proteinuria and leukocyturia (Table II).
Spearman analysis showed a slight negative correlation between serum magnesium and increased comorbidities (-0.26, p < 0.05) (Table III). Despite a negative correlation between serum magnesium and haematuria (-0.23, p < 0.05), increased comorbidities were not correlated with haematuria (0.12, p = 0.275).
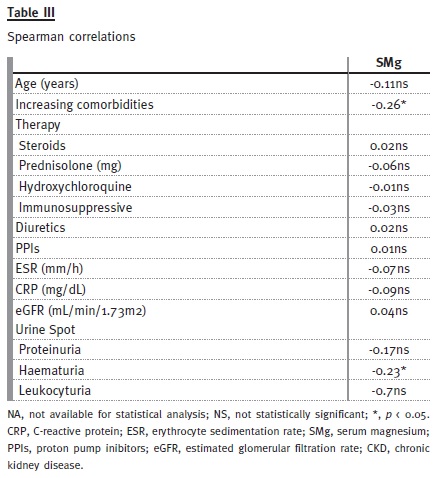
No significant correlations were found between serum magnesium and ESR, CRP, eGFR, proteinuria or leukocyturia (Table III).
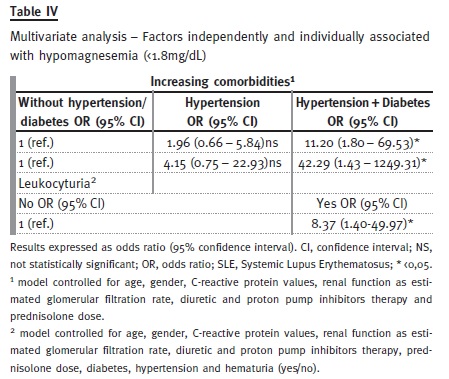
A multivariate analysis was performed to assess possible predictive factors for hypomagnesaemia (Table IV). Patients with hypertension+diabetes had a higher risk of hypomagnesaemia: OR 42.29 (95% CI 1.43-1249.31). Leukocyturia was also independently associated with hypomagnesaemia: OR 8.37 (95% CI 1.4 – 49.97). These results were controlled for confounding variables, such as age, gender, hypertension, diabetes, eGFR, CRP levels, haematuria and diuretic, PPI and prednisolone therapy. Haematuria was not found to be associated with hypomagnesaemia in the same model.
DISCUSSION
In our study hypomagnesaemia was frequent among SLE patients (20.6% of all patients), when comparing with general population. The prevalence of hypomagnesaemia in the general population range between 2.4% (American population between 55 and 64 years old) and 14.5% (unselected population group in Germany)24,25.
We hypothesised that the association of hypomagnesaemia with chronic inflammatory diseases should also be found in SLE. Lower serum magnesium could correlate with markers of inflammation, like CRP and ESR, which in turn correlate with disease activity in SLE patients17,18. However, we did not find a relationship between lower SMg and higher CRP or ESR. A possible explanation could be the non-inclusion of clinical variables that could better reflect disease activity and inflammation. The use of scales that measure disease activity incorporating clinical data, such as the SLEDAI score, could establish a clearer link between inflammation and magnesium.
The variables that we used to quantify inflammation (CRP and ESR) are also elevated in other clinical conditions, despite the major causes that have been excluded (neoplasia and infection).
In addition, serum magnesium concentration may not accurately reflect the total magnesium status.
Extracellular magnesium corresponds to 1% of the total body magnesium content, skeleton to 60% and intracellular to 39%. Serum magnesium only represents 0.3% of total magnesium. Normal serum magnesium concentration is maintained within normal range through regulation by kidneys, gastrointestinal tract and bone 26,27. In the early stages of magnesium depletion, blood magnesium concentration may still be within the normal range, whereas the concentration in urine may be significantly reduced27. In the PREVEND study, the authors did not find any association between serum magnesium and ischaemic heart disease but low urinary magnesium excretion was independently associated with a higher risk suggesting that urinary magnesium excretion may be a more useful marker of magnesium balance28,29.
In the early stages of CKD, the increase of magnesium excretion compensates the loss of renal function and serum levels are maintained in the normal range. If renal function deteriorates further (less than 30ml/min of creatinine clearance) excretion of magnesium tends to decrease26. Therefore, patients with CKD stages 4 and 5 were excluded from this analysis.
Additionally, low serum magnesium has been associated with the decline in kidney function in CKD patients independently from hypertension, diabetes and diuretic use30,31. Fractional magnesium excretion seems to be a sensitive parameter to detect tubule interstitial damage even before changes in the renal function occur32,33. Therefore, the evaluation of magnesium status in our study could be limited and the inclusion of urinary magnesium excretion could better reflect this subject.
Low serum magnesium has been associated with numerous conditions characterised by a chronic inflammatory status. Hypertension and diabetes have already been associated with hypomagnesaemia in several studies and higher SMg seems to be protective in cardiovascular diseases9,34,35. In our study, low serum magnesium was independently associated to hypertension and diabetes, regardless of CRP, diuretic therapy, prednisolone dose and eGFR.
The use of diuretics, in particular loop diuretics, increases the urinary excretion of magnesium36 but their use was not associated with lower serum magnesium.
Steroids are one of the most commonly used drugs to treat SLE but come with an expensive price in terms of side effects and long-term complications and their use in high doses for a significant period of time is associated with an increased cardiovascular risk37. We found that patients with hypertension and diabetes were taking higher daily dosages of prednisolone, but steroids therapy did not seem to influence serum magnesium. It seems that the presence of hypertension and diabetes influences serum magnesium, regardless of whether those comorbidities were a consequence of long-term steroid treatment.
Despite the influence of renal function in the regulation of serum magnesium, we did not find any association between eGFR and serum magnesium.
Additionally, eGFR did not influence the association of hypertension and diabetes with hypomagnesaemia, as already described.
Although we found an association between low serum magnesium and hypertension and diabetes, there is still a high percentage (15%) of patients with hypomagnesaemia not accounted for those comorbidities.
In the multivariate analysis, leukocyturia was found to be an independent predictor of lower serum magnesium.
Leukocyturia was related to lower serum magnesium, regardless of eGFR, hypertension or diabetes. In addition, haematuria had a negative correlation with serum magnesium that appears not to be related to an increased number of comorbidities.
Isolated haematuria and isolated pyuria are associated with active renal and non-renal disease in SLE patients38. Therefore, if haematuria and leukocyturia may represent higher SLE activity and lower serum magnesium is associated to inflammation in several diseases4-15, the association of low serum magnesium to haematuria and leukocyturia, may suggest that patients with lower levels of serum magnesium have higher (renal) disease activity. Furthermore, elevated urine magnesium excretion has been correlated with the magnitude of tubulo-interstitial fibrosis in glomerulonephropathies like lupus nephritis, although the correlation with serum magnesium and spot urine was not performed39.
In addition, there were other limitations to point out in our study. The small sample size may hinder a more robust analysis of the results and the crosssectional nature of the study does not allow establishing causality (and, therefore, whether hypomagnesaemia is cause or consequence of inflammation).
We could not confirm the absence of infection (or colonization) in all patients with leukocyturia.
Nevertheless, the recognition that serum magnesium is lower in a significant proportion of lupus patients should help promoting further investigation on this subject.
CONCLUSION
The presence of hypomagnesaemia in patients with SLE is high, especially when hypertension and diabetes are present. Nevertheless, we did not find an association between lower levels of serum magnesium and CRP or ESR. Increasing comorbidities and the presence of leukocyturia were independent predictors of lower serum magnesium. Finally, the association of lower serum magnesium levels with leukocyturia and haematuria may suggest a relationship with higher disease activity.
References
1. King DE. Inflammation and elevation of C-reactive protein: does magnesium play a key role? Magnes Res 2009; 22 (2): 57-59. [ Links ]
2. Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys 2007; 458(1): 48-56. [ Links ]
3. Weglicki WB. Hypomagnesemia and inflammation: clinical and basic aspects. Annu Rev Nutr 2012; 32:55-71. [ Links ]
4. Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 2011; 13(11):843-847. [ Links ]
5. Kupetsky-Rincon EA, Uitto J. Magnesium: novel applications in cardiovascular disease – a review of the literature. Ann Nutr Metab 2012; 61(2):102-110. [ Links ]
6. Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res 2010; 134(2):119-129. [ Links ]
7. Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2007; 2(2):366-373. [ Links ]
8. Srinivasan AR, Niranjan G, Kuzhandai Velu V, Parmar P, Anish A. Status of serum magnesium in type 2 diabetes mellitus with particular reference to serum triacylglycerol levels. Diabetes Metab Syndr 2012; 6(4):187-189. [ Links ]
9. Dasgupta A1, Sarma D, Saikia UK. Hypomagnesemia in type 2 diabetes mellitus. Indian J Endicrinol Metab 2012; 16(6):1000-1003. [ Links ]
10. Streja D, Cressey P, Rabkin SW. Associations between inflammatory markers, traditional risk factors, and complications in patients with type 2 diabetes mellitus. J Diabetes Complications 2003; 17(3):120-127. [ Links ]
11. Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol 2008; 19(1):50-56. [ Links ]
12. Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res 1995; 41(5):347-359. [ Links ]
13. Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res 2007; 20(2):107-129. [ Links ]
14. Guerrero-Romero F, Rodríguez-Morán M. Relationship between serum magnesium levels and C-reactive protein concentration, in non-diabetic, non-hypertensive obese subjects. Int J Obes Relat Metab Disord 2002; 26(4):469-474. [ Links ]
15. Nielsen FH. Effects of magnesium depletion on inflammation in chronic disease. Curr Opin Clin Nutr Metab Care 2014;17(6):525-530. [ Links ]
16. Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus – an update. Curr Opin Immunol 2012; 24(6):651-657. [ Links ]
17. Mok CC, Birmingham DJ, Ho LY, Hebert LA, Rovin BH. High-sensitivity C-reactive protein, disease activity, and cardiovascular risk factors in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2013; 65(3):441-447. [ Links ]
18. Stojan G, Fang H, Magder L, Petri M. Erythrocyte sedimentation rate is a predictor of renal and overall SLE disease activity 2013; 22(8):827-834.
19. Gafter-Gvili A, Leibovici L, Molad Y. Elevation of inflammatory markers in patients with systemic lupus erythematosus is associated with poorer outcome. Biomed Pharmacother 2013; 67(1):48-52. [ Links ]
20. Thomas JR. Magnesium Deficiency in Systemic Lupus Erythematosus. J Nutr Env Med 1997; 7(2):107-112.
21. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725. [ Links ]
22. Levey AS, Stevens LA, Schmid CH, et al. with the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9):604-612. [ Links ]
23. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39 (2 Suppl 1): S1-S266. [ Links ]
24. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med 2013; 126(3):256-263. [ Links ]
25. Schimatschek HF, Rempis R. Prevalente of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res 2001; 14(4):283-290. [ Links ]
26. Kanbay M, Yilmaz MI, Apetrii M, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol 2012; 36(3):228-237. [ Links ]
27. Fawcett WJ, Haxby EJ, Male DA: Magnesium: physiology and pharmacology. Br J Anaesth 1999; 83(2): 302–320. [ Links ]
28. Joosten MM, Gansevoort RT, Mukamal KJ, et al. for the PREVEND Study Group. Urinary and plasma magnesium and risk of ischemic heart disease. Am J Clin Nutr 2013; 97(6): 1299–1306. [ Links ]
29. Larsson SC. Urinary magnesium excretion as a marker of heart disease risk. Am J Clin Nutr 2013; 97(6): 1159–1160. [ Links ]
30. Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med 2013;126(9):825-831. [ Links ]
31. Tin A, Grams ME, Maruthur NM, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int 2015; 87(4):820-827. [ Links ]
32. Deekajorndech T. Fractional excretion magnesium (FE Mg) in systemic lupus erythematosus. J Med Assoc Thai 2005; 88(6):743-745. [ Links ]
33. Noiri C, Shimizu T, Takayanagi K, et al. Clinical significance of fractional magnesium excretion (FEMg) as a predictor of interstitial nephropathy and its correlation with conventional parameters. Clin Exp Nephrol 2015 Feb [Epub ahead of print]. [ Links ]
34. Rodríguez-Moran M, Guerrero-Romero F. Hypomagnesemia and prehypertension in otherwise healthy individuals. Eur J Intern Med 2014; 25(2):128-131. [ Links ]
35. Peters KE, Chubb SA, Davis WA, Davis TM. The relationship between hypomagnesemia, metformin therapy and cardiovascular disease complicating type 2 diabetes: the Fremantle Diabetes Study. PLoS One. 2013; 8(9): e74355 [ Links ]
36. Wile D. Diuretics: a review. Ann Clin Biochem 2012; 49(Pt 5):419-431. [ Links ]
37. Ansell BM. Overview of the side effects of corticosteroid therapy. Clin Exp Rheumatol 1991; 9(6):19-20. [ Links ]
38. Rahman P, Gladman DD, Ibanez D, Urowitz MB. Significance of isolated hematuria and isolated pyuria in systemic lupus erythematosus. Lupus 2001; 10(6):418-423. [ Links ]
39. Futrakul P, Yenrudi S, Futrakul N, et al. Tubular function and tubulointerstitial disease. Am J Kidney Dis 1999; 33(5): 886-891. [ Links ]
Drª Liliana Cunha
Department of Nephrology,
Hospital Prof. Dr. Fernando Fonseca
IC 19, 2720-276 Amadora, Portugal.
E-mail: liliana.goncalves.cunha@gmail.com
Conflict of interest statement: None declared.
Received for publication: 03/09/2015
Accepted in revised form: 19/10/2015














