Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.1 Lisboa mar. 2016
ORIGINAL ARTICLE
Peritoneal dialysis dropouts in different age and era cohorts: focus on the elderly
Causas de drop-out da diálise peritoneal em diferentes cohorts etários e em diferentes eras: foco no doente idoso
Andreia Campos1, Jorge Malheiro1,2, Laetitia Teixeira3, Sofia Santos1, M. João Carvalho1,2, António Cabrita1, Anabela Rodrigues1,2
1 Nephrology Department, Centro Hospitalar do Porto, Porto, Portugal
2 UMIB – Unit for Multidisciplinary Research in Biomedicine, Porto, Portugal
3 Instituto de Ciências Biomédicas Abel Salazar, Porto, Portugal
ABSTRACT
Introduction and Aims: Peritoneal dialysis (PD) is an efficient renal replacement therapy (RRT), but still remains underutilized at any age. Clinicians fear the rate of dropouts and lower technique survival, particularly in elderly patients. The authors aimed to explore such outcomes over the past 3 decades, in different age and era cohorts. Methods: Consecutive incident patients starting PD were identified from an ongoing registry-base prospective study of quality assessment. In order to control for an era effect, patients were assigned to 6 cohorts (5 years interval) according to the admission year between 1985 and 2014. Regression models taking competing risks into account were performed to identify potential prognostic factors for death and transfer to haemodialysis (HD) (adjusted for age, gender, diabetes, cohort era, automated peritoneal dialysis (APD) use, and first treatment modality – PD first, PD after HD, PD after renal transplant (RT). Then the patients were studied according to age at enrolment in the programme: A (18-44 years; n = 193) ; B (45-64 years; n = 176); C (≥ 65 years old; n = 75). The HD transfer rates using Poisson analysis were evaluated. The incidence of dropout rates was compared at different timesand between age groups, focusing particular attention on the elderly. Results: A total of 525 patients were evaluated: 211 male (40.2%), aged 48 ± 15.7 years old, on PD for 23 (IQR 9 – 41.5) months. The major cause of dropout technique was transfer to HD (35.4%), followed by renal transplantation (27.6%)and death (21.7%). The probability of technical failure and renal transplantation at 2 and 5 years was19.2% and 18.1% and 34.2%; 27.4%, respectively. Probability of death at 2 and 5 years was 12.7%, and 21.8%, respectively. The contemporary cohort was associated with a lower risk of mortality and lower risk of transfer to haemodialysis, with greater access to renal transplantation. The regression model Fine & Gray showed that older age was associated with increased mortality, but was not associated with greater technical failure. Transfer to HD occurred in the elderly at a rate of 11epy/100 patient-year (in comparison to 15 and 14 epy/100 patient-year in non-elderly groups A and B, respectively P = 0.33). The proportions of specific causes of technique failure did not change significantly according to age cohort. The dropoutrates due to access-related-infection and ultrafiltration failure decreased in the elderly group in the morecontemporary cohort, despite the differences were not statistically significant. Conclusions: The dropoutby technique failure decreased significantly in the recent decade. Age at admission in peritoneal dialysisdid not show to be a compromising factor of the technique survival.
Key-Words: Age; dropout; elderly; peritoneal dialysis; technique failure.
RESUMO
Introdução e Objetivos: A diálise peritoneal (DP) é uma técnica substitutiva da função renal (TSFR) com eficácia semelhante à hemodiálise (HD); no entanto, ainda permanece subutilizada em qualquer idade. Os médicos temem o elevado drop-out da técnica e a sua menor sobrevida, particularmente nos doentes idosos.
Os autores pretenderam explorar causas de drop-out e sobrevida da técnica ao longo das últimas três décadas, em diferentes faixas etárias, centrando uma atenção particular no idoso. Métodos: Foram usados dados do registo prospetivo do programa hospitalar de doentes adultos incidentes em DP. Tendo em conta as diferentes épocas, os doentes foram divididos em 6 coortes (5 anos de intervalo) de acordo com o ano de admissão no programa (1985-2014). Usaram-se modelos de regressão tendo em conta a análise de riscos competitivos para identificar potenciais fatores de prognóstico para a morte e transferência para HD (ajustados para a idade, género, diabetes, era, tipo de técnica e primeira modalidade de TSFR). Posteriormente, os doentes foram estudados de acordo com a idade à admissão do programa: A (18-44 anos); B (45-64 anos); C (≥ 65 anos de idade). Foram avaliadas as taxas de transferência para HD usando a análise de Poisson. As taxas de incidência de drop-out foram comparadas em diferentes épocas e entre os grupos etários, focando particular atenção no doente idoso. Resultados: Foram avaliados 525 pacientes: 211 eram do sexo masculino (40,2%), com idade média de 48 ± 15,7 anos, com follow-up mediano de 23 meses (IQR 9-41,5). A maior causa de drop-out da técnica foi a transferência para HD (35,4%), seguida do transplante renal (27,6%) e de morte (21,7%). A probabilidade de falência técnica e transplantação renal aos 2 e aos 5 anos foi 19,2% e 18,1% e 34,2%; 27,4%, respetivamente. A probabilidade de morte aos 2 e aos 5 anos foi 12,7%, e 21,8%, respetivamente.
A coorte mais recente associou-se a menor risco de mortalidade e menor transferência para HD. O modelo de regressão Fine & Gray mostrou que a idade avançada se associou a maior mortalidade, contudo não se associou a maior falência técnica. Nos idosos, a taxa de transferência para HD foi 11,2 episódios/100 doentes-ano (em comparação com 15 e 14 episódios/100 doentes-ano nos grupos A e B, respectivamente, p = 0,33). Não houve diferenças significativas nas causas de transferência para HD entre os diferentes grupos etários. As taxas de drop-out por falência de acessos e por falência de ultrafiltração diminuíram no grupo mais velho e na coorte mais recente, contudo as diferenças não foram estatisticamente significativas entre os grupos. Conclusões: O drop-out por falência técnica diminuiu significativamente na década mais recente. A idade de admissão na diálise peritoneal não mostrou ser um fator de comprometimento da sobrevida da técnica.
Palavras-Chave: Diálise peritoneal; drop-out; falência técnica; idosos; idade.
INTRODUCTION AND AIMS
Portugal is a leading European country concerning end-stage renal disease (ESRD) prevalence. The prevalence of renal replacement therapies (RRT) is reported as 1662 pmp in a recent ERA-EDTA registry annual report. Most of the patients are allocated to haemodialysis (HD) and only a minority (consistently less than 10%) is treated by peritoneal dialysis (PD). Nevertheless, there are a high number of kidney transplant patients1.
Previous studies showed that PD conferred higher early patient survival but this advantage was often lost in the long-term, with survival data depending much on treatment time and patient comorbidities including diabetes and age2. However, more recent investigation revealed that updated PD allows similar long-term patient survival in comparison with HD3,4.
These favourable contemporary trends were observed in elderly patients, also after controlling for the mode of planned/non-planned dialysis initiation5. In spite of this, PD still remains underutilized in any age group6. Peritoneal dialysis requires an appropriate living situation, some degree of mobility and vision, a peritoneum not disrupted by prior surgeries, and the ability to learn and independently perform a daily medical technique7,8. Barriers to PD can include medical and social factors, physician bias, late referral, and education not tailored to the needs of the patients, particularly in the older group.
In Portugal, by 2014, 59.1% of patients who started dialysis were over 65 years old and haemodialysis was the main RRT in these aged patients9. Older patients are less often allocated to PD, which is mainly related with their impairment to perform auto-dialysis and lack of a helper10. The development of assisted PD can overcome some of the barriers and enable frail older patients to have home-based dialysis treatment. Haemodialysis also poses challenges and inherent technical constraints in these patients with higher risk of hypotension and arrhythmia11 than the more physiologically gentle PD regimen. Besides, risk of cognitive loss and dementia was reported to be higher in HD, than under PD, in fully adjusted statistic models12. On the other hand, HD requires less technical participation, but there can be a substantial cost in time spent with the procedure and traveling to dialysis units, especially if transportation assistance is needed.
The dialysis procedure can also be particularly exhausting for elderly patients. With major clinical impact, vascular access can be difficult in this population implying repeated vascular procedures and complications7,8,11.
The survival of aging patients on dialysis improved over the last few years although survival studies remain controversial and there is no clear consensus concerning in which was the best RRT for elderly patients10,13,14-16. However, dialysis modality selection should not be solely dictated by survival comparisons that do not take into account treatment skills or complications, but also should consider patient preference. Considering technique survival clinicians often fear the rate of peritoneal infections and dropout in elderly patients. Loss of autonomy can be a limitation to maintain PD7.
This study aims to describe our centre experience in PD, exploring the technical survival and clinical outcomes in different age cohorts giving special attention to the elderly throughout the different decades, since the implementation of PD programme until now.
METHODS
Consecutive incident adult end-stage renal disease patients starting PD were identified from an ongoing registry-base prospective study of quality assessment.
Patients were initially tabulated in 5 cohorts between 1985 and 2014. Analysed variables included sex, underlying nephropathy, age at starting PD, major comorbidities, reason for PD (option/vascular access failure/other), previous renal replacement therapy (PD-first; PD after HD; PD after RT) and type of PD (CAPD, continuous ambulatory peritoneal dialysis or APD). Patient outcome was defined as the earliest event among: death, transfer to HD and renal transplantation. The follow-up was until 31 December 2014.
Survival analysis and regression models taking competing risks into account were performed in order to identify whether age was a prognostic factor for death and for transfer to HD, adjusting for gender, diabetes, cohort era, APD use, and first treatment modality (PD first, PD after HD, PD after RT).
Then, we explored rates of transfer to HD according to age at admission in three cohorts C1 (patients admitted in PD programme between 1985 and 1995); C2 (1996-2005) e C3 (2006-2014). The patients were divided into three groups considering age at PD start – A group (18 to 44 years old); B (45 to 64 years old) and C (65 or more years old).
Causes of transfer to HD were categorized as: access-related infection (peritonitis/exit site infection); catheter mechanic dysfunction (obstruction/ leak), ultrafiltration failure/underdialysis, non-compliance/ disability for self-dialysis, and other causes.
Dropout incidence rates and specific causes were compared between different cohort era and between different age groups with Poisson analysis.
All statistical analyses were performed using SPSS statistical software, version 14.0. A p value of 0.05 or less was considered statistically significant.
RESULTS
Survival analysis and age
A total of 525 patients were evaluated: 211 were male (40.2%), aged 48 ± 15.7 years old, staying on PD for median 23 (IQR 9 – 41.5) months (Table I). By exploring 5 intervals era cohorts, significantly variables characterizing the treated population were identified: PD as first treatment modality (p = 0.005), reason for PD (p = 0.001), number of diabetic patients (p = 0.042) and APD use (p = 0.005).
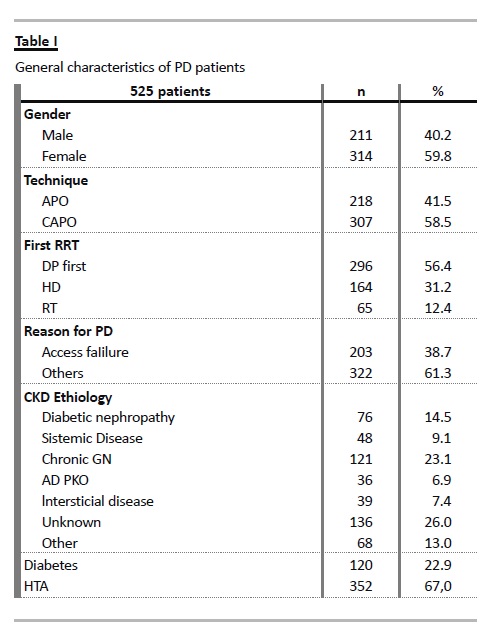
In the most recent cohort, PD-first occurred with the highest proportion (69.2%) with decreasing number of patients admitted after HD (15.4%) and in PD due to exhausted vascular access (14.1%). Also, more patients were treated with PD after failed RT. Diabetics admission decreased in intermediate era cohorts, but lately regained a proportion of 24.4%. Automated peritoneal dialysis use increased steadily reaching 65.2%, but decreased in the contemporary cohort to 44.9% (p < 0.001) (Table II).
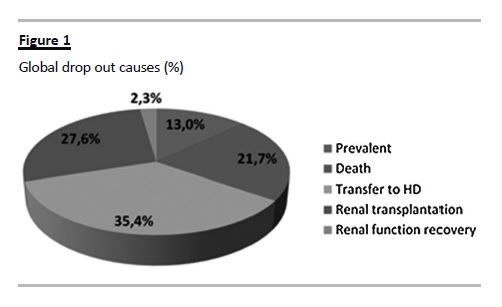
The global dropout causes were: transfer to HD (35.4%), death (21.7%), RT (27.6%) and recovery of renal function (2.3%) (Fig. 1). The proportion of technique failure due to infection (43.5%), ultrafiltration failure/underdialysis (22%) or mechanic complications (22%) did not change significantly according to the era cohort.
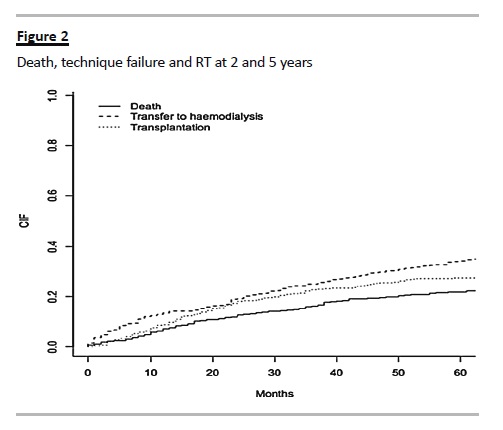
The probability of death, technique failure and RT at 2 years was 12.7%, 19.2% and 18.1% respectively; at 5 years of follow-up was 21.8%, 34.2% and 27.4% respectively (Fig. 2).
A Fine and Gray regression model showed that older age and diabetes are associated with a higher mortality risk but not technique failure. The contemporary five years cohort was associated with a lower risk of mortality and with a lower risk for transfer to HD, with higher access to RT.
Rates of transfer to HD by age groups
Peritoneal dialysis was chosen as an initial RRT in 48% of the elderly group (group C), less than in other age cohorts [53.4% of patients of the A group, 56.2% in B (p < 0.001)]. In the elderly group 60% of patients were women, 24% had diabetes and 65.3% had hypertension, percentages that did not differ significantly from other age groups; 52% came from HD after a mean time of five years. As expected, the death rate was higher and the transplantation rate was lower in the elderly. There were no significant statistical differences in the causes of death among different age groups.
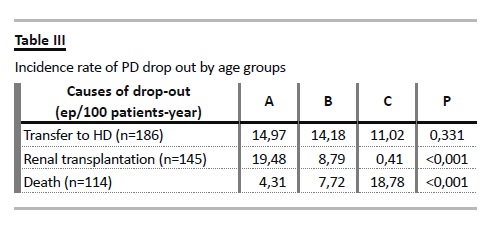
Notably, the incidence rate of dropout due to transfer to HD was not increased in the elderly group, but was marginally lower, at 11.02 episodes/100 patient year (Table III). There were no statistically significant differences in the proportions of causes of dropout for haemodialysis (infection, ultrafiltration failure/underdialysis, non-compliance/self-dialysis disability or mechanical complications) among different age groups (Table IV).
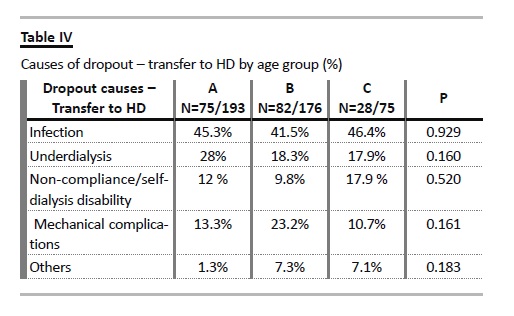
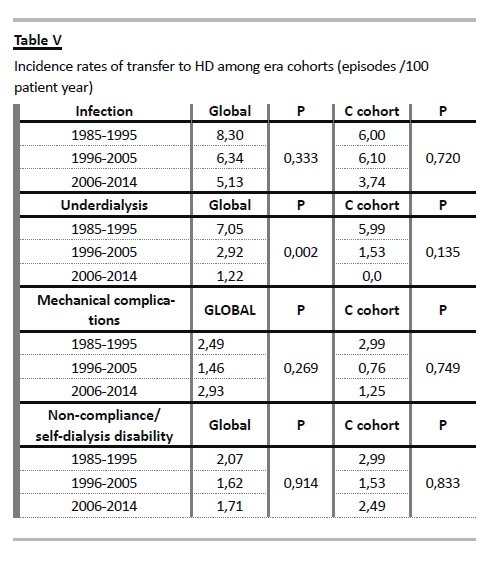
Exploring the rates of transfer to HD among era cohorts, favourable trends were documented, considering infection and ultrafiltration failure/inadequate dialysis, both in global population and in the elderly group (Table V). Except from the rate of transfer due to disability for self-dialysis, all the other causes of technique failure occurred at a lower rate in the elderly.
DISCUSSION AND CONCLUSIONS
In the present study we analysed technique and patient survival in our PD population by applying the more appropriate competing risk analysis, with a focus on the effect of age at admission. We achieved global good clinical outcomes in our programme which enabled a probability of technical failure, renal transplantation and death of 19.2% 18.1%, 12.7% at 2 years and 34.2%; 27.4%, 21.8% at 5 years, respectively.
Notably, dropout due to technique failure decreased in contemporary PD, after adjustment for relevant clinical variables. It is known that PD technique survival varies throughout the world depending upon access to transplantation, haemodialysis and centre practices. In our centre, with active promoter of cadaveric and live donor renal transplantation, the access to renal transplantation in younger cohorts is a competing event to include in survival analysis and a relevant factor to valorise in integrated patient care.
Focusing on the elderly group, despite being the largest and fastest growing group of patients on dialysis, they are still less likely to be started on PD17. In Portugal, by the end of 2014, PD was less used irrespective of patient age; it was the first RRT in only 8.73% (n = 216) of total of patients who started dialysis (n = 2223); 20.8% were over 65 years old9. In the United Kingdom, when the RRT was analysed by age – less than 65 and 65 years or more – 36 and 18% of incident patients, respectively, were on PD. In Canada, 12% of patients over the age of 75 years started on PD. In contrast, in France, where assisted PD using community nurses have been available for many years, PD is predominantly a treatment of the elderly with 54% of men and 59% of women on PD being over 70 years of age1,18-20. In our global PD programe only around 15 % were older than 65.
Death was, as expected, the main reason for dropout in the elderly group, mainly due to cardiovascular disease and infections. It is known that PD spares the aged patient from the vascular instrumentation and associated risks, contrary to HD procedures.
The fundamental physiology of PD is not age-dependent – a continuous ultrafiltration avoids volume shifts and haemodynamic complications, such as myocardial stunning and cerebral ischaemia21-23. However, we did not find significant statistical differences in death causes among different age groups. Higher mortality in elderly PD patients due to peritonitis has been described, presumably because age and comorbidities menace the immune response and the recovery from an acute insult24. On the other hand, facing that elderly patients have a higher incidence of intestinal pathology, including diverticulosis, bowel perforation, and constipation, menacing PD performance17,25 our prophylactic protocols shown to overcome such risk as evidenced by similar technique survival in comparison with the younger patients.
In our experience, older age at start of PD was not a determinant of lower technique failure. Moreover, the incidence rate of drop out to HD was lower in the elderly when compared with other groups, contrary to what one might expect. Studies comparing technique failure between younger and elderly PD patients remain inconsistent26-28. In some studies, the greater risk of technique failure in elderly compared with younger PD patients has been attributed to social reasons, including inability to perform PD because of cognitive or mechanical reasons; but risk of infection-related technique failure was similar26,29- 31. A retrospective study by Yang et al. demonstrated that the mean death-censored technique survival was similar between younger and elderly PD patients (74.4 months and 64.5 months, respectively in patients 64.9 years of age and younger and 75 years of age or older, p = 0.78)30. Conflicting results are much dependent on centre effect and specific treatment skills. In our study, rates of access-related infections, catheter dysfunction, ultrafiltration failure/underdialysis were lower in elderly patients; only disability was a major cause of transfer to HD in the elderly. Assisted PD (with a family helper) is used in a minority of our patients (< 15%). A broader nurse team assisted PD regimen would overcome this. Unfortunately this is not our reality. Data from the French Language Peritoneal Dialysis Registry, including 9822 incident patients started PD between January 2002 and December 2010 concluded that, compared with patients undergoing self-care PD, those with assisted PD had a lower risk for transfer to haemodialysis32.
Interestingly, the dropout by ultrafiltration failure/inadequate dialysis decreased significantly in recent decades. These aspects translate technique advances (such as standard use of solutions with low glucose degradation products, automated PD, optimized volume control and peritonitis prophylaxis) and cumulative experience of a multidisciplinary team in providing better healthcare with special focus on the elderly patient.
We acknowledge that the chosen age cut-off to categorize the elderly group is nowadays a critical issue: most reports of outcomes of dialysis in the aged patients are retrospective and use varied definitions of elderly which can be a limitation in data analysis, but we used a cut-off of 65 or more years old as standard used in epidemiological studies.
Nephrologists often face the challenge of providing care to much older patients with variable comorbidities25.
More than the chronological age, the functional status and frailty score should preferably be considered as the variables that dictate the prognosis in both HD and PD33.
To conclude, our study shows that dropout and technique survival in elderly under PD were not poor as compared with other age groups. Therefore, it supports that PD is feasible in higher number of older patients. However, the rate of transfer to HD due to disability for self-dialysis was put on evidence. In the absence of a technique helper, challenging barriers to self-dialysis still remain: cardiovascular comorbidity, impaired vision, deafness, poor mobility, and cognitive problems. Besides these, older patients are more often socially isolated, live in poor accommodation, have financial problems, and they can be psychologically depressed due to loss of autonomy. The PD option for the elderly needs to be reconsidered in light of the increasing number of older people requiring dialysis, as placing the majority on HD will be a huge financial burden to any health-care system10.
The French experience of community-based PD shows that this can be achieved and sustainable. A recent Canadian study shows also that development of homecare assistance can significantly increase the number of patients using PD34-36.
Facing our experience in treating this group of patients, we reinforce that allocation to dialysis modalities should be individualized, according to the family environment, after discussion on whether this should be realized in hospital or home-based, and if there is no medical contraindication to the technique.
Older patients should not be denied to choose the dialysis modality that better suits their lifestyle and expectations, particularly facing their limited life.
Additionally, contrary to the general assumption, there is no dramatic difference in clinical outcomes in elderly patients who are on PD versus those on HD. Quality of life (QOL) seems to be at least as good.
BOLDE (Broadening Options for Long-term Dialysis in the Elderly) shows in two closely matched demographic groups of older dialysis patients, that QOL was similar, if not better, in those on PD, with less treatment intrusion34.
Multi-disciplinary team approaches need to be adopted to promote strategies that improve PD uptake, including assisted exchanges, use of automated PD, reductions in the number of exchanges by including icodextrin in the PD treatment. It is necessary to understand the factors that contribute to the reluctance of elderly patients to consider PD, as well as clinician bias35-38.
We believe that home care assistance could allow more elderly patients to receive PD, minimizing transportation costs and improving the delivery of health care to this special and growing population.
References
1. Noordzij M, Kramer A, Abad Diez JM, et al. Renal replacement therapy in Europe: a summary of the 2011 ERA-EDTA Registry Annual Report. Clin Kidney J 2014;7(2):227-238. [ Links ]
2. Vonesh EF, Moran J. Mortality in end-stage renal disease: a reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 1999;10(2):354-365. [ Links ]
3. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 2012; 27(9):3568-3575. [ Links ]
4. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensitymatched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010; 21(3):499-506. [ Links ]
5. Heaf JG, Wehberg S. Relative survival of peritoneal dialysis and haemodialysis patients: effect of cohort and mode of dialysis initiation. PLoS ONE 2014; 9(3): e90119. [ Links ]
6. Noordzij M, Jager KJ. Survival comparisons between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 2012;27(9):3385-3387 [ Links ]
7. Dimkovic N, Oreopoulos DG. Chronic peritoneal dialysis in the elderly: a review. Perit Dial Int 2000; 20(3):276–283. [ Links ]
8. Castrale C, Evans D, Verger C, et al. Peritoneal dialysis in elderly patients: report from the French Peritoneal Dialysis Registry (RDPLF). Nephrol Dial Transplant 2010;25(1):255-262. [ Links ]
9. Relatório anual 2014. Gabinete de Registo do tratamento da Doença Renal Terminal. Sociedade Portuguesa de Nefrologia. [ Links ]
10. Brown EA. Peritoneal dialysis in elderly patients: clinical experience. Perit Dial Int 2005;25 Suppl 3:S88-91. [ Links ]
11. Brown EA. Peritoneal dialysis for older people: overcoming the barriers. Kidney Int Suppl 2008;(108):S68-71. [ Links ]
12. Wolfgram DF, Szabo A, Murray AM, Whittle J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 2015;35(2):189-198. [ Links ]
13. Joshi U, Guo Q, Yi C, et al. Clinical outcomes in elderly patients on chronic peritoneal dialysis: a retrospective study from a single center in China. Perit Dial Int 2014;34(3):299-307 [ Links ]
14. Genestier S, Meyer N, Chantrel F, et al. Prognostic survival factors in elderly renal failure patients treated with peritoneal dialysis: a nine-year retrospective study. Perit Dial Int 2010;30(2):218-226. [ Links ]
15. Sakaci T, Ahbap E, Koc Y, et al. Clinical outcomes and mortality in elderly peritoneal dialysis patients. Clinics (Sao Paulo) 2015; 70(5):363-368. [ Links ]
16. Han SS, Park JY, Kang S, et al. Dialysis modality and mortality in the elderly: A metaanalysis. Clin J Am Soc Nephrol 2015; 10(6):983-993. [ Links ]
17. Jassal SV, Watson D. Dialysis in late life: benefit or burden. Clin J Am Soc Nephrol 2009;4(12):2008-2012. [ Links ]
18. The Renal Association. UK Renal Registry Report 2003. [ Links ]
19. Canadian Organ Replacement Registry. Treatment of End-Stage Organ Failure in Canada: 2002 and 2003. Canadian Institute for Health Information: Ottawa, Ontario, 2005. [ Links ]
20. Verger C, Ryckelynck JP, Duman M, et al. French peritoneal dialysis registry (RDPLF): outline and main results. Kidney Int Suppl 2006; (103): S12–S20. [ Links ]
21. Morelle J, Goffin E. Peritoneal dialysis for stroke: amazing, but promising! Perit Dial Int 2014;34(1):7-8. [ Links ]
22. Godino Mdel C, Romera VG, Sánchez-Tomero JA, et al. Amelioration of ischemic brain damage by peritoneal dialysis. J Clin Invest 2013;123(10):4359-4363. [ Links ]
23. Wolfgram DF, Szabo A, Murray AM, Whittle J.. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int 2015;35(2):189-198. [ Links ]
24. Lim WH, Dogra GK, McDonald SP, Brown FG, Johnson DW. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int 2011;31(6):663-671. [ Links ]
25. Franco MR, Fernandes NM. Dialysis in the elderly patient: a challenge of the XXI century–narrative review. J Bras Nefrol 2013;35(2):132-141 [ Links ]
26. De Vecchi AF, Maccario M, Braga M, Scalamogna A, Castelnovo C, Ponticelli C. Peritoneal dialysis in nondiabetic patients older than 70 years: comparison with patients aged 40 to 60 years. Am J Kidney Dis 1998; 31(3):479–490. [ Links ]
27. Holley JL, Bernardini J, Perlmutter JA, Piraino B. A comparison of infection rates among older and younger patients on continuous peritoneal dialysis. Perit Dial Int 1994; 14(1): 66–699. [ Links ]
28. Baek MY, Kwon TH, Kim YL, Cho DK. CAPD, an acceptable form of therapy in elderly ESRD patients: a comparative study. Adv Perit Dial 1997; 13:158–161. [ Links ]
29. Dimkovic N, Oreopoulos DG. Assisted peritoneal dialysis as a method of choice for elderly with end-stage renal disease. Int Urol Nephrol 2008; 40(4):1143–1150. [ Links ]
30. Yang X, Fang W, Kothari J, et al. Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: experiences from one center and a review of the literature. Int Urol Nephrol 2007; 39(4):1295–1302. [ Links ]
31. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012; 27(9):3568-3575. [ Links ]
32. Lobbedez T, Verger C, Ryckelynck JP, Fabre E, Evans D. Is assisted peritoneal dialysis associated with technique survival when competing events are considered? Clin J Am Soc Nephrol. 2012;7(4):612-618. [ Links ]
33. Alfaadhel TA, Soroka SD, Kiberd BA, Landry D, Moorhouse P, Tennankore KK. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015;10(5):832-840. [ Links ]
34. Brown EA, Johansson L, Farrington K, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010;25(11):3755-3763. [ Links ]
35. Oliver MJ, Quinn RR. Chapter 23: Assisted Peritoneal Dialysis in Elderly Persons. Geriatric Nephrology Curriculum. American Society of Nephrology, 2009.
36. Osterlund K, Mendelssohn D, Clase C, Guyatt G, Nesrallah G.Identification of facilitators and barriers to home dialysis selection by Canadian adults with ESRD. Semin Dial 2014; 27(2):160-172. [ Links ]
37. Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW. with the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in the Netherlands. Am J Kidney Dis 2004; 43(5):891–899. [ Links ]
38. Hiramatsu M on behalf of the Japanese Society for Elderly Patients on Peritoneal Dialysis. How to improve survival in geriatric peritoneal dialysis patients. Perit Dial Int 2007; 27(Suppl 2):S185–189. [ Links ]
Drª Andreia Cristiana Santos Ferreira Dias Campos
Nephrology Department, Centro Hospitalar do Porto – Hospital Geral de Santo António
Largo Prof. Abel Salazar 4099-001 Porto, Portugal.
E-mail: andcriscampos@hotmail.com
Disclosure of Potential Conflicts of Interest: None declared.
Received for publication: 29/06/2015
Accepted in revised form: 6/11/2015














