Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.1 Lisboa mar. 2016
CASE REPORT
Urinothorax – an uncommon complication following renal transplantation: a case report
Urinotórax – uma complicação rara após o transplante renal: caso clínico
Joel Ferreira, Rita Gouveia, Ana Mateus, Pedro Cruz, Carlos Oliveira, José M. Carvalho, Maria J. Ferreira, Aura Ramos
Department of Nephrology, Renal Transplantation Unit, Hospital Garcia de Orta, Almada, Portugal.
ABSTRACT
Urinothorax is an uncommon cause of pleural effusion, mostly related to urinary tract injury or obstructive uropathy. Several pathophysiological mechanisms have been proposed to explain the transdiaphragmatic evasion of urine from an intra-abdominal collection known as urinoma. Biochemical analysis of pleural fluid and imagiological studies are essential for diagnosis, which requires a high index of suspicion. Treatment directed to the underlying cause and aimed at reducing intra-urinary pressure is sufficient in most cases. We report herein the case of a urinothorax in a young kidney transplant recipient. An early urinary leak occurred through an infarction area of the kidney graft. Successful conservative treatment involved thoracocentesis and prolonged double-J stenting and Foley catheterization.
Key-Words: Kidney transplantation; pleural effusion; urinoma.
RESUMO
O urinotórax constitui uma causa rara de derrame pleural, associando-se geralmente a lesões do trato urinário ou à uropatia obstrutiva. Vários mecanismos fisiopatológicos foram propostos para explicar o extravasamento transdiafragmático de urina a partir de uma colecção intra-abdominal conhecida como urinoma. A análise bioquímica do líquido pleural e meios complementares de imagem são fundamentais para o diagnóstico que requer um elevado grau de suspeição. Um tratamento direcionado à causa subjacente, com o objectivo de promover o alívio da pressão no trato urinário, é geralmente suficiente na maioria dos casos. Apresenta-se o caso de um urinotórax num jovem transplantado renal, que apresentou um leak urinário precoce, numa área de enfarte do enxerto renal. Uma abordagem conservadora envolvendo uma toracocentese, a manutenção do stent duplo J e uma algaliação prolongada, permitiu a cicatrização bem sucedida do leak urinário.Palavras-Chave: Derrame pleural; transplante renal; urinoma.
INTRODUCTION
Urinothorax refers to the accumulation of urine in the pleural space and constitutes a very rare cause of pleural effusion. Since its first description, in 1968, from the studies of Corriere et al. on ureteral obstruction in dogs1, fewer than 70 cases have been reported worldwide until today. Most of them were related to urinary tract trauma or obstructive uropathy2. The first two cases of urinothorax in renal transplant recipients were described in 1985 and were attributed to obstruction at the ureterovesical junction3.
We present a case of urinothorax in a renal transplant recipient, secondary to urinoma from a urinary leak, which is one of the most common urological complications in the early post-transplantation period.
CASE REPORTA 22-year-old Black male underwent a cadaveric renal transplant in May 2014. He was on haemodialysis since February 2011 because of end-stage renal disease secondary to reflux nephropathy. The vesicoureteral reflux (VUR) had been surgically corrected at the age of 4 years and was excluded by a recent retrograde and voiding urethrocystography prior to transplantation. His past medical history also included a right inguinal hernioplasty in childhood and arterial hypertension. Pre-transplantation panel reactive antibody (PRA) was 4%.
The patient received a kidney from a 62-year-old deceased donor, dead from cranioencephalic trauma, with normal renal function and diagnosis of arterial hypertension. This was a full HLA antigen (A, B and DR) mismatch transplantation. The surgery proceeded without complications; the left kidney with one artery and one vein was placed in the right iliac fossa of the recipient and a Lich Gregoir ureteroneocystostomy was performed with placement of a double-J ureteral stent.
Cold-ischaemia time was 20 hours and 20 minutes. The induction immunosuppressive regimen used was thymoglobulin (1mg/kg/day), mycophenolate mofetil (1000 mg bid), steroids (methylprednisolone 500mg bolus followed by prednisolone 20 mg /day) and delayed introduction of tacrolimus (0.15mg/kg/day).
After kidney transplant, diuresis resumed immediately and there was a progressive decline in serum creatinine values. A MAG-3 renogram, routinely performed within 48 hours, showed a renographic curve consistent with increased intraparenchymal transit time suggesting acute tubular necrosis and a parenchymal lesion area with hypocaptation in the lower third of the kidney allograft.
On day 2 post-transplant, after the third administration of thymoglobulin, the patient developed an acute pulmonary oedema (APO), which resolved with intravenous diuretic therapy. On the 4th administration, thymoglobulin-related APO relapsed in association with fever, and progressed to respiratory failure and need for non-invasive ventilatory support for 24 hours. Therefore, it was decided to stop thymoglobulin therapy. A chest radiograph showed bilateral interstitial infiltrates and, given the suspicion of superimposed respiratory infection, despite the absence of condensation on imaging, piperacillin and tazobactam were started. Blood and urine cultures were performed and proved to be negative.
Pulmonary embolism was excluded by a ventilation/ perfusion scintigraphy. On day 7, Foley catheter removal was followed by reduction of diuresis, worsening of graft function (serum creatinine increased from 2.8 to 4.3 mg/dL), and painful graft at examination.
Another renogram was performed which excluded urinary leak on delayed imaging at 3 hours and showed a possible intracapsular perigraft collection.
A Doppler ultrasound revealed a slight ureterohydronephrosis, increased resistance in renal artery, and did not reveal any perigraft collection or vascular thrombosis. Tacrolimus blood levels were within the targeted therapeutic values (8-12 ng/mL). Given the presumption of an eventual cellular rejection, 500mg/day methylprednisolone pulses were started for 5 days. On the same day, the Foley catheter was replaced and the patient resumed haemodialysis because of fluid overload.
On day 10, the patient still complained of severe graft pain, abdominal distension, and was dependent on renal replacement therapy. A graft biopsy was performed. Given the lack of response to corticosteroids and persistent anuria, acute humoral rejection was suspected so plasma exchange therapy and intravenous immunoglobulin (ivIg) were started.
The kidney histology was compatible with cellular rejection type IA of Banff classification and donor specific antibodies (DSA) were negative. Therefore, humoral rejection therapy was discontinued. The Folley catheter and central venous catheter were removed on day 15.
After a transient improvement on renal graft function, the patient developed anuria, ascitis, acute massive right pleural effusion documented on chest radiograph (Fig. 1), fever and raised inflammatory markers. Meropenem and vancomycin empiric antibiotherapy was started in order to treat a presumptive pulmonary infection and metapneumonic pleural effusion. Blood and urine cultures were again negative.
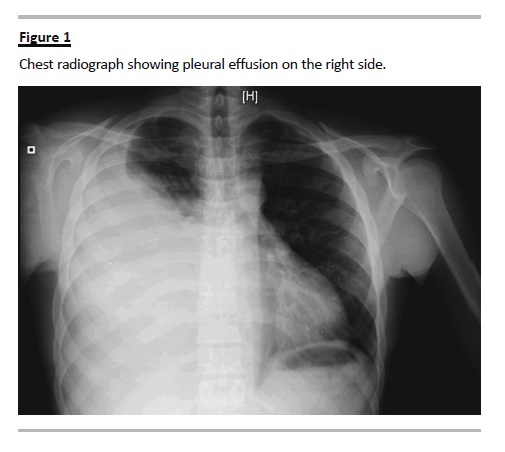
Prompt thoracocentesis was performed, with drainage of a 1700cc yellow fluid. A non-contrastenhanced thoraco-abdominal computed tomography (CT) scan confirmed an extensive right pleural effusion, and showed a subcapsular and hypodense triangular area on the kidney graft possibly accounting for an eventual parenchymal infarct zone, significant intraperitoneal fluid, and liquid collection in the right retroperitoneal space (Fig. 2). The biochemical examination of pleural fluid was compatible with a transudate according to Lights criteria and revealed an elevated creatinine (10.6 mg/dL), with a pleural-to-serum creatinine ratio greater than 1 (serum creatinine 5.6mg/dL), consistent with the diagnosis of urinothorax. Pleural fluid culture was negative. The Foley catheter was placed in order to reduce the urinary tract pressure.
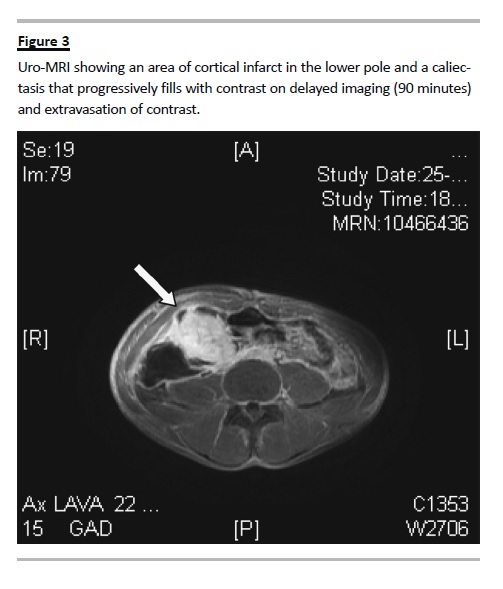
Further exploration through MAG3 renogram showed a radionuclide hypocaptation in the lower third of the kidney graft and, in delayed imaging, it revealed a urinary leak in the lower third of the kidney, possibly at the ureterovesical junction. The uro-magnetic resonance imaging (MRI) showed an area of cortical infarct in the lower pole by involvement of the posterior subsegmental arteriole, and a caliectasis
which progressively filled with contrast on delayed imaging, and extravasation of contrast towards the peri-renal urinoma; the ureteropelvic junction had no complications (Fig. 3).
Foley catheterization was continued for a further 10 days, with progressive improvement and stabilization of the kidney allograft function (serum creatinine 1.4 mg/dL). The patient was discharged on the 38th day post-transplant.
Two weeks after Foley catheter removal, a comparative uro-MRI revealed worsening of the urinoma, which was also consistent with the elevation of serum creatinine. Therefore, the Foley catheter was again replaced and maintained for 6 weeks more.
In subsequent imaging performed 3 months later, the urinoma was practically solved, without any evidence of contrast-enhanced urine leakage, and renal function stabilized (serum creatinine 2mg/dL).
DISCUSSION
Urine leakage is the most common urological complication following renal transplant, occurring in less than 10% of procedures5. It manifests usually more than one week after the surgery6 but can occur until the third month of post-transplantation7. Urinary leak is suggested by onset of graft dysfunction associated with decreased urine output, anuria or urine drainage from the wound. Signs and symptoms can also include lower abdominal pain8, fever and graft tenderness.
Therefore, urinary leak has to be considered in the differential diagnosis of conditions that manifest by sudden onset of graft pain and dysfunction, like acute rejection, thrombosis or obstruction by other fluid collections.
The most frequent site of leakage is the ureteroneocystostomy that can arise from necrosis of the distal ureter9, but it can also occur at the kidney allograft level. However, it is a rare condition and only a few cases of urinary leakage caused by segmental renal allograft infarction have been reported worldwide. The incidence of segmental infarction may vary from 4% to 42% and generally arises from disruption or thrombosis of the renal arterial branches10.
The retroperitoneal leakage of urine resulting from a urinary tract leak is known as urinoma. Common causes of urinoma formation include complications of surgical procedures in the native kidney or the ureter, retroperitoneal inflammation, trauma, urinary tract obstruction and malignant disease11. The resultant retroperitoneal collection can find a way across transdiaphragmatic barrier and cause a transudative pleural effusion called urinothorax12.
Concerning aetiology, most of urinothoraces are related to accidental or iatrogenic urinary tract trauma, stone or other obstruction (including congenital malformation), extra-urinary malignancy like Hodgkins disease, and, it might also occur, following therapeutic procedures like shock wave lithotripsy or percutaneous nephrolithotomy12. As previously mentioned, the first two published cases of urinothorax in a renal transplant recipient were attributed to obstruction at ureterovesical junction3 and the other one to ureteral perforation in a paediatric patient4.
Two theories have been proposed regarding mechanisms responsible for the transdiaphragmatic evasion of urine: (1) urine may travel trough lymphatic drainage into pleural space or (2) retroperitoneal urine firstly enters the peritoneal cavity and afterwards collections directly spread to the thorax across the diaphragm due to excess pressure13.
Diagnosis of urinothorax essentially relies on a high index of suspicion. Chest radiograph shows pleural effusion ipsilateral with the urinoma, and rarely there are cases of bilateral or contralateral urinothoraces11.
If the clinical context is suggestive, a thoracocentesis and pleural fluid biochemical analysis are very helpful in establishing the diagnosis. Classically, urinothorax is considered to be a transudate14 but high levels of lactate dehydrogenase in pleural fluid are frequent so that it may be misclassified as an exsudate15. The fluid is typically yellow, clear and has a distinctive ammonia smell13,16. Other more inconstant features include low glucose level and low pH. However, the most significant biochemical parameter remains a pleural fluid-to-serum creatinine ratio higher than 1.
Complementary radiologic explorations are also fundamental in establishing the diagnosis. Common findings on abdominal and thoracic CT scan include renal or other excretory tract pathology, the presence of perirenal urinomas, or the extravasation of contrast-enhanced urine into the retroperitoneum or pleural space17. Renal scintigraphy with the use of technetium-99m labeled diethylenetriamine pentaacetic acid (DTPA) has been used in the past to reveal any extravasation of urine from the site of the leak11. MAG-3 scans are now the preferred study by virtue of better resolution. More invasive studies such as retrograde pyelogram and endoscopy of the renal collecting system should be used when there is a possibility of a therapeutic intervention17.
Concerning our patient, the diagnosis was reached because visualization of a potential urinoma on CT scan led us to determine the creatinine level in pleural fluid. Indeed, at first glance, the patient could have other more evident causes of pleural effusion, such as fluid overload or pulmonary infection. Uro-MRI was crucial to confirm and to identify the source of the leak since contrast was not used on CT scan and therefore delayed images were not obtained. The infarction area was already seen on the first renogram but not on the Doppler ultrasound even though it is not the gold standard test. The second renogram did not reveal any urinary leak on images obtained after 3 hours, so the leak might not be present at that time or was confined to the subcapsular space. In addition, the initial improvement of graft function could not allow us to predict the occurrence of a leak at this site.
Treatment of urinothorax is relatively straightforward and should be directed to the correction of the underlying cause, which is sufficient in most cases18.
When pleural effusion persists and patients are very symptomatic, drainage trough a thoracic tube is recommended.
Regarding specifically renal allograft recipients, initial management of urine leakage should include: Foley catheter and double-J stent placement (if not already in place); percutaneous nephrostomy to divert urine and facilitate healing; with or without percutaneous drainage of the urinoma9, 19. Severe urinomas must indeed be drained percutaneously in order to reduce the risk of infection and extrinsic ureteral compression. Periodic ureteropyelograms should be performed and once the leak has resolved, the nephrostomy tube and Foley catheter are removed.
On the other hand, ureteral stent should be maintained for 4 to 6 weeks more19. Ultimately, if the leak persists and when ureteroneocistostomy is the site of the leakage, open surgical techniques may be needed to reimplant the ureter9. However, in the absence of contraindications, primary endourological management proved to be successful in most of cases20.
The capacity of routine stent placement during renal transplantation to prevent urine leak remains controversial since studies results are conflicting19.
A randomized study of 194 patients found the urinary leak rate to be higher in unstented compared to stented patients (6 vs. 1%)21. In another randomized study of 280 patients, there was no difference in ureteral obstruction or leak rates between stented and unstented recipients (3.5 vs. 6.6%; p = 0.23)22.
As far as allograft segmental infarction is concerned, treatment depends on the size of the infarction area, and when extensive it usually involves the removal of the transplanted kidney. However when the lesion is restricted to the upper or the lower pole, partial nephrectomy proved to be successful10, and when the area involved is smaller or diagnosis has been delayed, conservative treatment is indicated in the majority of patients since blood loss during surgery might further worsen graft function23.
In this case our surgical team opted for a conservative approach with replacement of the Foley catheter.
Ascitis resolved spontaneously and did not require any drainage. However, healing time was prolonged and the urinoma recurred early after decatheterization.
Therefore, when leakage is not at the site of ureteroneocistostomy, one should recommend a longer duration of catheterization.
In conclusion, urinothorax is a rare but treatable cause of pleural effusion and its diagnosis requires a high index of suspicion. In renal allograft recipients, when other most plausible causes are discarded, one should suspect of the possibility of extravasation of urine into the pleural space. Its occurrence is usually ipsilateral to a massive urinoma. Endourological intervention with pressure relief, and eventually urine derivation trough percutaneous nephrostomy, are sufficient in most cases to provide healing of the leakage.
References
1. Corriere JN, Miller WT, Murphy JJ. Hydronephrosis as a cause of pleural effusion. Radiology 1968;90(1):79-84. [ Links ]
2. Bhattacharya A, Venkataramarao SH, Kumar S, Mittal BR. Urinothorax demonstrated on 99mTc ethylene dicysteine renal scintigraphy. Nephrol Dial Transplant 2007; 22(6):1782-1783. [ Links ]
3. Carcillo J, Salcedo JR. Urinothorax as a manifestation of nondilated obstructive uropathy following renal transplantation. Am J Kidney Dis. 1985;5(3):211-213. [ Links ]
4. Kees-Folts D, Cole BR. Ureteral urine leak presenting as a pleural effusion in a renal transplant recipient. Pediatr Nephrol 1998;12(8):666-667. [ Links ]
5. Nie ZL, Zhang KQ, Li QS, Jin FS, Zhu FQ, Huo WQ. Treatment of urinary fistula after kidney transplantation. Transplant Proc 2009;41(5):1624–1626. [ Links ]
6. Humar A, Matas AJ. Surgical complications after kidney transplantation. Semin Dial 2005;18(6):505-510. [ Links ]
7. Rao PS, Ravindran A, Elsamaloty H, Modi KS. Emphysematous urinoma in a renal transplant patient. Am J Kidney Dis 2001;38(5):E29. [ Links ]
8. Dirlik A, Erinc R, Ozcan Z, et al. Diagnosis of urinary leakage in renal transplant patients: ultrasonographic, clinical and scintigraphic findings. Official Journal of the Turkish Society of Nephrology 2001;10(4):239-243. [ Links ]
9. Richard HM. Perirenal transplant fluid collections. Semin Intervent Radiol 2004; 21(4):235-237. [ Links ]
10. Salehipour M, Roozbeh J, Eshraghian A, et al. Postrenal transplant urinary leakage caused by segmental infarction of a renal allograft treated by partial nephrectomy. Exp Clin Transplant 2011; 9(2):153-155. [ Links ]
11. Laskaridis L, Kampantais S, Toutziaris C, et al. Urinothorax – an underdiagnosed cause of acute dyspnea: report of a bilateral and of an ipsilateral urinothorax case. Case Rep Emerg Med 2012;2012: doi: 10.1155/2012/395653. [ Links ]
12. Batura D, Haylock-Vize P, Naji Y, Tennant R, Fawcett K. Management of iatrogenic urinothorax following ultrasound guided percutaneous nephrostomy. J Radiol Case Rep 2014;8(1):34-40. [ Links ]
13. Chandra A, Pathak A, Kapur A, Russia N, Bhasin N. Urinothorax: a rare cause of severe respiratory distress. Indian J Crit Care Med 2014;18(5):320-322. [ Links ]
14. Stark DD, Shades JG, Baron RL, Koch DD. Biochemical features of urinothorax. Arch Intern Med 1982;142(8):1509-1511. [ Links ]
15. Garcia-Pachon E, Padilla-Navas I. Urinothorax: case report and review of the literature with emphasis on biochemical diagnosis. Respiration 2004;71(5): 533-536. [ Links ]
16. Ferreira PG, Furriel F, Ferreira AJ. Urinothorax as an unusual type of pleural effusion – Clinical report and review. Rev Port Pneumol 2013;19(2):80-83. [ Links ]
17. Wei B, Takayama H, Bacchetta M. Urinothorax: an unusual cause of pleural effusion. Respir Med CME 2009;2:179-180. [ Links ]
18. Salcedo JR. Urinothorax: report of 4 cases and review of the literature. J Urol 1986;135(4):805-808. [ Links ]
19. Duty BD, Conlin MJ, Fuchs EF, Barry JM. The current role of endourologic management of renal transplantation complications. Adv Urol 2013;2013:246520. [ Links ]
20. Matalon TA, Thompson MJ, Patel SK, Ramos MV, Jensik SC, Merkel FK. Percutaneous treatment of urine leaks in renal transplantation patients. Radiology 1990;174(3 Pt2): 1049–1051. [ Links ]
21. Benoit G, Blanchet P, Eschwege P, Alexandre L, Bensadoun H, Charpentier B. Insertion of a double pigtail ureteral stent for the prevention of urological complications in renal transplantation: a prospective randomized study. J Urol 1996;156(3): 881–884. [ Links ]
22. Dominguez J, Clase CM, Mahalati K, et al. Is routine ureteric stenting needed in kidney transplantation? A randomized trial. Transplantation 2000;70(4):597–601. [ Links ]
23. Kanchanabat B, Siddins M, Coates T et al. Segmental infarction with graft dysfunction: an emerging syndrome in renal transplantation? Nephrol Dial Transplant 2002; 17: 123-128. [ Links ]
Dr. Joel Ferreira
Nephrology Department, Hospital Garcia de Orta
Avenida Torrado da Silva, 2801-951 Almada, Portugal.
E-mail: joel-ferreira@sapo.pt
Disclosure of Potential Conflicts of Interest: None declared.
Received for publication: 20/08/2015
Accepted in revised form: 21/10/2015














